
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
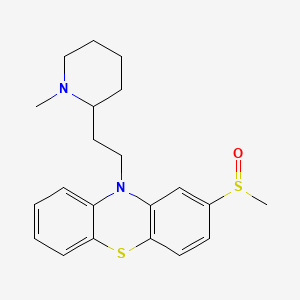

1. Serentil
1. Serentil
2. 5588-33-0
3. Lidanar
4. Calodal
5. Lidanil
6. Thioridazine-2-sulfoxide
7. Tps23
8. Tps-23
9. Thioridazine Thiomethyl Sulfoxide
10. Nc-123
11. Mesoridazina
12. Mesoridazinum
13. Thd-2-so
14. T-2-so
15. Tps 23
16. Mesoridazine Free Base
17. Nsc186066
18. Thioridazien Thiomethyl Sulfoxide
19. Nsc 186066
20. Nsc-186066
21. 10-(2-(1-methyl-2-piperidyl)ethyl)-2-methylsulfinyl Phenothiazine
22. Chembl1088
23. 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-(methylsulfinyl)-10h-phenothiazine
24. 10h-phenothiazine, 10-(2-(1-methyl-2-piperidinyl)ethyl)-2-(methylsulfinyl)-
25. 5xe4nwm740
26. Chebi:6780
27. 10-(2(1-methyl-2-piperidyl)ethyl)-2-(methylsulfinyl)-phenothiazine
28. 10-[2-(1-methylpiperidin-2-yl)ethyl]-2-methylsulfinylphenothiazine
29. 10-(2-(1-methyl-2-piperidinyl)ethyl)-2-(methylsulfinyl)-10h-phenothiazine
30. 10h-phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylsulfinyl)-
31. Nsc 186066; Serentil; Tps 23
32. Ncgc00163157-01
33. 5588-33-0 (free Base)
34. 10-(2(1-methyl-2-piperidyl)ethyl)-2-(methylsulfinyl)phenothiazine
35. Dsstox_cid_3265
36. 10-(2-(1-methylpiperidin-2-yl)ethyl)-2-(methylsulfinyl)-10h-phenothiazine
37. 2-methanesulfinyl-10-[2-(1-methyl-piperidin-2-yl)-ethyl]-10h-phenothiazine
38. Dsstox_rid_76948
39. Dsstox_gsid_23265
40. Mesoridazinum [inn-latin]
41. Mesoridazina [inn-spanish]
42. 10-[2(1-methyl-2-piperidyl)ethyl]-2-(methylsulfinyl)phenothiazine
43. 10-[2-(1-methyl-2-piperidyl)ethyl]-2-methylsulfinyl Phenothiazine
44. Lidanar (tn)
45. Cas-5588-33-0
46. Thioridazine Monosulfoxide Analog
47. Hsdb 3357
48. Thioridazine 2-sulfoxide
49. Mesoridazine (usan/inn)
50. Unii-5xe4nwm740
51. Sr-01000759425
52. Mesoridazine [usan:inn:ban]
53. Prestwick1_000529
54. Prestwick2_000529
55. Prestwick3_000529
56. Mesoridazine [mi]
57. Mesoridazine [inn]
58. Biomol-nt_000013
59. Mesoridazine [hsdb]
60. Mesoridazine [usan]
61. Mesoridazine [vandf]
62. Schembl19735
63. Bspbio_000517
64. Mesoridazine [mart.]
65. Mesoridazine [who-dd]
66. Spbio_002438
67. Bpbio1_000569
68. Bpbio1_001167
69. Gtpl7227
70. Dtxsid3023265
71. Hy-b1482a
72. Hms2090h22
73. Tox21 112018
74. Tox21_110052
75. Tox21_112018
76. Bdbm50131440
77. Ns-531
78. Phenothiazine, 10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylsulfinyl)-
79. Tox21_110052_1
80. Db00933
81. Ncgc00014529-01
82. Ncgc00163157-02
83. Ncgc00163157-03
84. Ncgc00163157-04
85. Ncgc00163157-05
86. Nci60_001547
87. Ab00513854
88. Cs-0013666
89. Ft-0671034
90. Thioridazine Impurity B [ep Impurity]
91. C07143
92. D02671
93. Ab00513854-11
94. Ab00513854_12
95. Ab00513854_13
96. L000852
97. Q6821618
98. Sr-01000759425-4
99. Wln: T C666 Bn Isj Eso&1 B2- Bt6ntj A
100. Brd-a14395271-001-01-5
101. Brd-a14395271-074-03-8
102. Thioridazine Hydrochloride Impurity B [ep Impurity]
103. 10-[2-(1-methyl-2-piperidyl)ethyl]-2-methylsulfinyl-phenothiazine
104. 2-methanesulfinyl-10-[2-(1-methylpiperidin-2-yl)ethyl]-10h-phenothiazine
| Molecular Weight | 386.6 g/mol |
|---|---|
| Molecular Formula | C21H26N2OS2 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 386.14865580 g/mol |
| Monoisotopic Mass | 386.14865580 g/mol |
| Topological Polar Surface Area | 68.1 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 502 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipsychotic Agents, Phenothiazine; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
THE ANTIPSYCHOTIC DRUGS HAVE A HIGH THERAPEUTIC INDEX & ARE REMARKABLY SAFE AGENTS. FURTHERMORE, MOST PHENOTHIAZINES HAVE RELATIVELY FLAT DOSE-RESPONSE CURVE AND CAN BE USED OVER WIDE RANGE OF DOSAGES. ...SIDE EFFECTS ARE OFTEN EXTENSIONS OF MANY PHARMACOLOGICAL ACTIONS OF DRUGS... /PHENOTHIAZINES/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 414
...INDICATED FOR MGMNT OF SCHIZOPHRENIA, ORG BRAIN DISORDERS, SYMPTOMS OF ALC WITHDRAWAL, & PSYCHONEUROSES. CLINICAL STUDIES TO DATE INDICATE THAT MESORIDAZINE BESYLATE HAS LOW INCIDENCE OF ADVERSE REACTIONS COMPARED WITH OTHER PHENOTHIAZINES. /BESYLATE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1026
8 WK THERAPY WITH THIORIDAZINE & MESORIDAZINE BESYLATE. THIORIDAZINE TREATED PT WERE SUPERIOR TO LATTER TREATED PT. IMPLICATIONS FOR CHEMOTHERAPY OF SCHIZOPHRENICS CONSIDERED.
GILLIS, DAVIS; EFFECTS OF THIORIDAZINE AND MESORIDAZINE ON THE INTERPERSONAL LEARNING OF ACUTE SCHIZOPHRENICS; CURR THER RES CLIN EXP 21 507 (1977)
For more Therapeutic Uses (Complete) data for MESORIDAZINE (6 total), please visit the HSDB record page.
NEUROLEPTIC AGENTS ... SHOULD BE USED WITH EXTREME CAUTION, IF @ ALL, IN UNTREATED EPILEPTIC PT & IN PT UNDERGOING WITHDRAWAL FROM CENTRAL DEPRESSANT DRUGS SUCH AS ALCOHOL, BARBITURATES, OR BENZODIAZEPINES. MOST ANTIPSYCHOTIC DRUGS ... CAN BE USED SAFELY IN EPILEPTICS IF MODERATE DOSES ARE ATTAINED GRADUALLY AND IF CONCOMITANT ANTICONVULSANT DRUG THERAPY IS MAINTAINED. /PHENOTHIAZINES/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 408
PHENOTHIAZINES INHIBIT EJACULATION WITHOUT INTERFERING WITH ERECTION. /PHENOTHIAZINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 161
WT GAIN & INCR IN APPETITE OCCUR WITH ALL PHENOTHIAZINES... PERIPHERAL EDEMA OCCURS IN 1-3% OF PT & MAY BE OF ENDOCRINE ORIGIN. /PHENOTHIAZINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 162
The antipsychotic drugs are not addicting ... However, some degree of physical dependence may occur, with malaise and difficulty in sleeping developing several days after abrupt discontinuation. Tolerance usually develops to the sedative effects ... over a period of days or weeks. Tolerance ... is demonstrable in behavioral and biochemical experiments in animals ... /Antipsychotic drugs/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 413
For more Drug Warnings (Complete) data for MESORIDAZINE (20 total), please visit the HSDB record page.
Used in the treatment of schizophrenia, organic brain disorders, alcoholism and psychoneuroses.
Mesoridazine, the besylate salt of a metabolite of thioridazine, is a phenothiazine tranquilizer. Pharmacological studies in laboratory animals have established that mesoridazine has a spectrum of pharmacodynamic actions typical of a major tranquilizer. In common with other tranquilizers it inhibits spontaneous motor activity in mice, prolongs thiopental and hexobarbital sleeping time in mice and produces spindles and block of arousal reaction in the EEG of rabbits. It is effective in blocking spinal reflexes in the cut and antagonizes d-amphetamine excitation and toxicity in grouped mice. It shows a moderate adrenergic blocking activity in vitro and in vivo and antagonizes 5-hydroxytryptamine in vivo. Intravenously administered, it lowers the blood pressure of anesthetized dogs. It has a weak antiacetylcholine effect in vitro.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AC - Phenothiazines with piperidine structure
N05AC03 - Mesoridazine
Absorption
Well absorbed from the gastrointestinal tract.
TOTAL URINARY & FECAL EXCRETION BY MONKEYS (10 DAY PERIOD) OF THIORIDAZINE WAS 64-76% & 83-92% FOR MESORIDAZINE (FECAL EXCRETION 2-4 TIMES GREATER). GREATER EXCRETION OF LATTER MAY BE DUE TO LESSER OVERALL TISSUE ADSORPTION OR LESS EXTENSIVE ENTERO-HEPATIC CYCLING.
FORREST ET AL; COMMUN PSYCHOPHARMACOL 3(5) 325 (1979)
WELL ABSORBED FROM GI TRACT. ONSET & DURATION OF ACTION & METABOLIC FATE... NOT...PRECISELY DETERMINED /HUMAN, ORAL, IM/. IN ANIMAL STUDIES, APPROX 2/3 OF DOSE...EXCRETED IN FECES VIA BILE & 1/3 OF DOSE...EXCRETED IN URINE. /BESYLATE/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1970
PHENOTHIAZINES CROSS PLACENTAL BARRIER & MAY APPEAR IN MILK OF NURSING MOTHERS...PHENOTHIAZINES &...METABOLITES...EXCRETED IN URINE, BILE, & FECES. CERTAIN METABOLITES &...FREE DRUGS...DETECTED IN URINE UP TO 6 MO AFTER THERAPY...DISCONTINUED /HUMAN, ORAL, IM/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1970
PHENOTHIAZINES...ABSORBED WELL FROM GI TRACT & FROM PARENTERAL SITES. GENERALLY...CLEARED FROM PLASMA WITHIN APPROX 3 HR...DISTRIBUTED TO MOST BODY TISSUES...HIGH CONCN OF UNCHANGED DRUG...IN BRAIN...METABOLITES PREDOMINATE IN LUNG, LIVER, KIDNEYS...SPLEEN /HUMAN, ORAL, IM/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1970
THIORIDAZINE-2-SULFOXIDE PROBABLY YIELDS THIORIDAZINE-2,5-DISULFOXIDE IN RAT: ZEHNDER, K ET AL, BIOCHEM PHARMAC, 11, 535 (1962). /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. T-13
Thioridazine 2-sulfoxide is a known human metabolite of Thioridazine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
24 to 48 hours
Based upon animal studies, mesoridazine, as with other phenothiazines, acts indirectly on reticular formation, whereby neuronal activity into reticular formation is reduced without affecting its intrinsic ability to activate the cerebral cortex. In addition, the phenothiazines exhibit at least part of their activities through depression of hypothalamic centers. Neurochemically, the phenothiazines are thought to exert their effects by a central adrenergic blocking action.
...MECHANISM OF ACTION OF ANTIPSYCHOTIC DRUGS WITH RESPECT TO THERAPEUTIC EFFICACY & SIDE EFFECTS MAY RELATE TO INHIBITION OF DOPAMINE ACTIVATION OF ADENYLATE CYCLASE. /PHENOTHIAZINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 160
ABOUT THIS PAGE
62
PharmaCompass offers a list of Mesoridazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mesoridazine manufacturer or Mesoridazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mesoridazine manufacturer or Mesoridazine supplier.
PharmaCompass also assists you with knowing the Mesoridazine API Price utilized in the formulation of products. Mesoridazine API Price is not always fixed or binding as the Mesoridazine Price is obtained through a variety of data sources. The Mesoridazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mesoridazine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mesoridazine, including repackagers and relabelers. The FDA regulates Mesoridazine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mesoridazine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Mesoridazine supplier is an individual or a company that provides Mesoridazine active pharmaceutical ingredient (API) or Mesoridazine finished formulations upon request. The Mesoridazine suppliers may include Mesoridazine API manufacturers, exporters, distributors and traders.
Mesoridazine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mesoridazine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mesoridazine GMP manufacturer or Mesoridazine GMP API supplier for your needs.
A Mesoridazine CoA (Certificate of Analysis) is a formal document that attests to Mesoridazine's compliance with Mesoridazine specifications and serves as a tool for batch-level quality control.
Mesoridazine CoA mostly includes findings from lab analyses of a specific batch. For each Mesoridazine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mesoridazine may be tested according to a variety of international standards, such as European Pharmacopoeia (Mesoridazine EP), Mesoridazine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mesoridazine USP).
