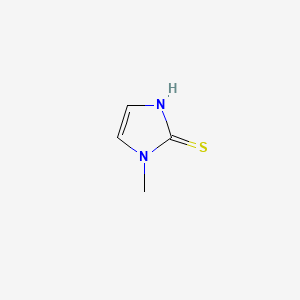

1. 1 Methyl 2 Mercaptoimidazole
2. 1-methyl-2-mercaptoimidazole
3. Favistan
4. Henning, Thiamazol
5. Hexal, Thiamazol
6. Mercasolyl
7. Mercazol
8. Mercazole
9. Mercazolyl
10. Merkazolil
11. Methizol
12. Methylmercaptoimidazole
13. Methymazol
14. Metisol
15. Metizol
16. Strumazol
17. Tapazole
18. Thiamazol Henning
19. Thiamazol Hexal
20. Thiamazole
21. Thimazol
22. Thyrozol
23. Tiamazol
24. Tirodril
1. 60-56-0
2. Thiamazole
3. Tapazole
4. 2-mercapto-1-methylimidazole
5. 1-methylimidazole-2-thiol
6. Mercazolyl
7. Methimazol
8. Favistan
9. Mercazole
10. Metazolo
11. Thymidazol
12. Thymidazole
13. 1-methyl-1h-imidazole-2-thiol
14. Mercaptazole
15. Merkazolil
16. Metothyrin
17. Metothyrine
18. Strumazol
19. Thiamazol
20. Thycapzol
21. Basolan
22. Danantizol
23. Frentirox
24. Merkastan
25. Metotirin
26. Thacapzol
27. Thycapsol
28. Metizol
29. Mercasolyl
30. Methylmercaptoimidazole
31. 1-methyl-2-mercaptoimidazole
32. Methiamazole
33. 2h-imidazole-2-thione, 1,3-dihydro-1-methyl-
34. 3-methyl-1h-imidazole-2-thione
35. Usaf El-30
36. N-methyl-2-mercaptoimidazole
37. Tapuzole
38. Thimazole
39. 1-methyl-1,3-dihydro-2h-imidazole-2-thione
40. 1-methyl-2-imidazolethiol
41. 1-methyl-1h-imidazole-2(3h)-thione
42. 2-mercaptomethylimidazole
43. 1,3-dihydro-1-methyl-2h-imidazole-2-thione
44. 1-methylimidazole-2(3h)-thione
45. Imidazole-2-thiol, 1-methyl-
46. 1-metylo 2 Merkaptoimidazolem
47. Mercaptizole
48. 1-methyl-1,3-dihydroimidazole-2-thione
49. 4-imidazoline-2-thione, 1-methyl-
50. Mfcd00179321
51. Thiamazole [inn]
52. Methimazole (usp)
53. Methimazole [usp]
54. Nsc-38608
55. Tiamazol
56. Chembl1515
57. Mercazolylum
58. Methimazolum
59. Metimazol
60. 1-methyl-2,3-dihydro-1h-imidazole-2-thione
61. Chebi:50673
62. Imidazole, 1-methyl-2-mercapto-
63. 554z48xn5e
64. Nsc38608
65. 223768-14-7
66. Cas-60-56-0
67. Tiamazolo [dcit]
68. Ncgc00016273-01
69. Methamazole
70. Thiamazolum
71. Strumazole
72. Tiamazolo
73. Dsstox_cid_820
74. Thiamazol [inn-french]
75. Tiamazol [inn-spanish]
76. Dsstox_rid_75808
77. Dsstox_gsid_20820
78. Thiamazolum [inn-latin]
79. 85916-84-3
80. Mmz
81. Felimazole
82. Tapazole (tn)
83. Smr000058376
84. 1-metylo 2 Merkaptoimidazolem [polish]
85. Hsdb 3361
86. Sr-05000001672
87. Einecs 200-482-4
88. Nsc 38608
89. 1-methyl-imidazole-2-thiol
90. Tiamazole
91. Unii-554z48xn5e
92. Ai3-60285
93. 1,3-dihydro-1-methyl-2h-imidazol-2-thione
94. Thiamazole,(s)
95. 2-mercapto-1-methyl-1h-imidazole
96. Prestwick_1010
97. Spectrum_000995
98. Methimazole [mi]
99. Thiamazole [jan]
100. Prestwick0_000786
101. Prestwick1_000786
102. Prestwick2_000786
103. Prestwick3_000786
104. Spectrum2_001273
105. Spectrum3_000495
106. Spectrum4_000048
107. Spectrum5_000954
108. M0868
109. Methimazole [hsdb]
110. Methimazole [iarc]
111. Thiamazole (jp17/inn)
112. 1-methylimidazole-2-thione
113. Methimazole [vandf]
114. Methimazole-d3(methyl-d3)
115. N-methyl Imidazole-2-thiol
116. Thiamazole [mart.]
117. 2-mecapto 1-methylimidazole
118. 2-mercapto-1-methylimidazol
119. Thiamazole [who-dd]
120. 2-mercapto-n-methylimidazole
121. Schembl41647
122. 2-mercapto-3-methylimidazole
123. Bspbio_000892
124. Bspbio_001989
125. Kbiogr_000515
126. Kbioss_001475
127. Methimazole [usp-rs]
128. Mls000028413
129. Mls002548853
130. Bidd:gt0163
131. Divk1c_000188
132. Spectrum1500396
133. Wln: T5n Cnj A Bsh
134. 1-methyl-2-mercapto-imidazole
135. 2-mercapto-1-methyl-imidazole
136. Spbio_001266
137. Spbio_002831
138. 1-methyl-2-mercapto Imidazole
139. Imidazole-2-thio, 1-methyl-
140. Bpbio1_000982
141. Gtpl6649
142. Methimazole, Analytical Standard
143. Dtxsid4020820
144. Methimazole (tapazole, Northyx)
145. 1-methyl-1h-immidazole-2-thiol
146. 1-methyl-3h-imidazole-2-thione
147. Hms500j10
148. Kbio1_000188
149. Kbio2_001475
150. Kbio2_004043
151. Kbio2_006611
152. Kbio3_001489
153. Methimazole [orange Book]
154. Thiamazole [ep Monograph]
155. 1-methyl-1-h-imidazole-2-thiol
156. Ninds_000188
157. Hms1570m14
158. Hms1920l17
159. Hms2090b17
160. Hms2091d12
161. Hms2094c05
162. Hms2097m14
163. Hms3259l09
164. Hms3651i13
165. Hms3714m14
166. Pharmakon1600-01500396
167. Methimazole [usp Monograph]
168. 1-methyl-1h-imidazole-2-thiol #
169. Amy11202
170. Bcp02147
171. Hy-b0208
172. Str03572
173. Zinc1187543
174. Tox21_110341
175. Tox21_201341
176. Tox21_300532
177. Ac-785
178. Bdbm50241361
179. Ccg-39656
180. Nsc757111
181. S1609
182. Stk300018
183. Stk802184
184. Akos000119427
185. Akos000269708
186. Tox21_110341_1
187. Bs-3743
188. Ccg-220786
189. Db00763
190. Nc00636
191. Nsc-757111
192. 2-mercapto-1-methylimidazole, >=99%
193. Idi1_000188
194. Ncgc00016273-02
195. Ncgc00016273-03
196. Ncgc00094721-01
197. Ncgc00094721-02
198. Ncgc00094721-03
199. Ncgc00094721-04
200. Ncgc00094721-05
201. Ncgc00094721-06
202. Ncgc00094721-07
203. Ncgc00178875-01
204. Ncgc00254307-01
205. Ncgc00258893-01
206. Methimazol 100 Microg/ml In Acetonitrile
207. Sbi-0206922.p001
208. Sbi-0206922.p004
209. Db-053649
210. 2h-imidazole-2-thione,3-dihydro-1-methyl-
211. Ft-0603253
212. Sw197088-3
213. C07190
214. D00401
215. H10722
216. Ab00443630-03
217. Ab00443630-04
218. Ab00443630_06
219. Ab00443630_07
220. Methimazole, Vetranal(tm), Analytical Standard
221. A832780
222. Q419663
223. Sr-01000695434
224. Q-201364
225. Sr-01000695434-2
226. Sr-05000001672-1
227. Sr-05000001672-2
228. Brd-k54416256-001-15-7
229. Z57901905
230. F0001-2396
231. F1679-0258
232. Thiamazole, European Pharmacopoeia (ep) Reference Standard
233. Methimazole, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 114.17 g/mol |
|---|---|
| Molecular Formula | C4H6N2S |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 114.02516937 g/mol |
| Monoisotopic Mass | 114.02516937 g/mol |
| Topological Polar Surface Area | 47.4 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 119 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Methimazole |
| PubMed Health | Methimazole (By mouth) |
| Drug Classes | Antithyroid Agent |
| Drug Label | Methimazole (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from the drugs of the thiouracil series primarily because it has a 5- instead of a 6-membered ring.Each tablet contains 5... |
| Active Ingredient | Methimazole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Cedar Pharms; Sun Pharm Inds; Sandoz; Emcure Pharms Usa; Mylan |
| 2 of 4 | |
|---|---|
| Drug Name | Tapazole |
| PubMed Health | Methimazole (By mouth) |
| Drug Classes | Antithyroid Agent |
| Drug Label | TAPAZOLE (Methimazole Tablets, USP) (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from the drugs of the thiouracil series primarily because it has a 5- instead of a 6-membered r... |
| Active Ingredient | Methimazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | King Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Methimazole |
| PubMed Health | Methimazole (By mouth) |
| Drug Classes | Antithyroid Agent |
| Drug Label | Methimazole (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from the drugs of the thiouracil series primarily because it has a 5- instead of a 6-membered ring.Each tablet contains 5... |
| Active Ingredient | Methimazole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 5mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Cedar Pharms; Sun Pharm Inds; Sandoz; Emcure Pharms Usa; Mylan |
| 4 of 4 | |
|---|---|
| Drug Name | Tapazole |
| PubMed Health | Methimazole (By mouth) |
| Drug Classes | Antithyroid Agent |
| Drug Label | TAPAZOLE (Methimazole Tablets, USP) (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from the drugs of the thiouracil series primarily because it has a 5- instead of a 6-membered r... |
| Active Ingredient | Methimazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | King Pharms |
Antithyroid Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
METHIMAZOLE IS APPROX 10 TIMES AS POTENT AS PROPYLTHIOURACIL & IS MORE PROMPT IN ELICITING ANTITHYROID RESPONSE. DRUG ALSO EXHIBITS MORE PROLONGED ACTION THAN PROPYLTHIOURACIL...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 911
...USED IN TREATMENT OF HYPERTHYROIDISM...(1) AS DEFINITIVE TREATMENT, TO CONTROL DISORDER IN ANTICIPATION OF SPONTANEOUS REMISSION IN GRAVE'S DISEASE; (2) IN CONJUNCTION WITH RADIOIODINE, TO HASTEN RECOVERY WHILE AWAITING EFFECTS OF RADIATION; & (3) TO CONTROL DISORDER IN PREPN FOR SURGICAL TREATMENT. /ANTITHYROID DRUGS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1400
THERE ARE NO COMMERCIAL PREPN AVAIL FOR PARENTERAL USE IN RARE EVENT THAT TREATMENT CANNOT BE GIVEN BY MOUTH. FOR THIS EVENTUALITY & FOR EXPTL PURPOSES, FREELY WATER-SOL COMPD, METHIMAZOLE, CAN BE DISSOLVED IN SALINE SOLN & STERILIZED BY HEAT.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1412
Methimazole /is/ indicated in the treatment of hyperthyroidism, including prior to surgery or radiotherapy, and as adjunct in the treatment of thyrotoxicosis or thyroid storm. Propylthiouracil may be preferred over methimazole for use in thyroid storm, since propylthiouracil inhibits peripheral conversion of thyroxine (T4) to triiodothyronine (T3). /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 436
...WOMEN TAKING THESE AGENTS SHOULD NOT BREAST-FEED THEIR INFANTS. /ANTITHYROID DRUGS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1413
BECAUSE AGRANULOCYTOSIS CAN DEVELOP RAPIDLY, PERIODIC WHITE-CELL COUNTS ARE OF LITTLE HELP. PATIENTS SHOULD IMMEDIATELY REPORT DEVELOPMENT OF SORE THROAT OR FEVER, WHICH USUALLY HERALDS ONSET OF THIS REACTION.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1399
MAIN DRAWBACK TO THERAPY WITH ANTITHYROID DRUGS IS HIGH INCIDENCE OF RELAPSE WHEN TREATMENT IS STOPPED. ... FREQUENT TAKING OF MEDICATION FOR LONG PERIODS OF TIME IS ANOTHER DISADVANTAGE &, ALTHOUGH UNTOWARD REACTIONS...ARE NOT FREQUENT & RARELY SERIOUS, THEY CONSTITUTE FURTHER DISADVANTAGE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1414
CROSS SENSITIVITY TO OTHER THIOAMIDE DERIVATIVES MAY OCCUR IN SUSCEPTIBLE PT.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 611
For more Drug Warnings (Complete) data for METHIMAZOLE (13 total), please visit the HSDB record page.
In the United States, methimazole is indicated for the treatment of hyperthyroidism in patients with Graves' disease or toxic multinodular goiter for whom thyroidectomy or radioactive iodine therapy are not appropriate treatment options. Methimazole is also indicated for the amelioration of hyperthyroid symptoms in preparation for thyroidectomy or radioactive iodine therapy. In Canada, methimazole carries the above indications and is also indicated for the medical treatment of hyperthyroidism regardless of other available treatment options.
Methimazole inhibits the synthesis of thyroid hormones resulting in an alleviation of hyperthyroidism. Onset of action occurs within 12 to 18 hours, and its duration of action is 36 to 72 hours, likely due to concentration of methimazole and some metabolites within the thyroid gland after administration. The most serious potential side effect of methimazole therapy is agranulocytosis, and patients should be instructed to monitor for, and report, any signs or symptoms of agranulocytosis such as fever or sore throat. Other cytopenias may also occur during methimazole therapy. There also exists the potential for severe hepatic toxicity with the use of methimazole, and monitoring for signs and symptoms of hepatic dysfunction, such as jaundice, anorexia, pruritus, and elevation in liver transaminases, is prudent in patients using this therapy.
Antithyroid Agents
Agents that are used to treat hyperthyroidism by reducing the excessive production of thyroid hormones. (See all compounds classified as Antithyroid Agents.)
H03BB02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
H - Systemic hormonal preparations, excl. sex hormones and insulins
H03 - Thyroid therapy
H03B - Antithyroid preparations
H03BB - Sulfur-containing imidazole derivatives
H03BB02 - Thiamazole
Absorption
Absorption of methimazole after oral administration is rapid and extensive, with an absolute bioavailability of approximately 0.93 and a Tmax ranging from 0.25 to 4.0 hours. Cmax is slightly, but not significantly, higher in hyperthyroid patients, and both Cmax and AUC are significantly affected by the oral dose administered.
Route of Elimination
Urinary excretion of unchanged methimazole has been reported to be between 7% and 12%. Elimination via feces appears to be limited, with a cumulative fecal excretion of 3% after administration of methimazole. Enterohepatic circulation also appears to play a role in the elimination of methimazole and its metabolites, as significant amounts of these substances are found in the bile post-administration.
Volume of Distribution
The apparent volume of distribution of methimazole has been reported as roughly 20 L. Following oral administration, methimazole is highly concentrated in the thyroid gland - intrathyroidal methimazole levels are approximately 2 to 5 times higher than peak plasma levels, and remain high for 20 hours after ingestion.
Clearance
Following a single intravenous bolus injection of 10mg of methimazole, clearance was found to be 5.70 L/h. Renal impairment does not appear to alter clearance of methimazole, but patients with hepatic impairment showed a reduction in clearance roughly proportional to the severity of their impairment - moderate insufficiency resulted in a clearance of 3.49 L/h, while severe insufficiency resulted in a clearance of 0.83 L/h. There does not appear to be any significant differences in clearance based on thyroid status (i.e. no difference between euthyroid and hyperthyroid patients).
FOUR DAYS AFTER IV ADMIN OF (14)C-METHIMAZOLE TO RATS, RETENTION OF (14)C WAS GREATEST IN THYROID & ADRENALS; 76% OF DOSE HAD BEEN EXCRETED IN URINE & 6% IN FECES.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 114
(14)C-METHIMAZOLE RADIOACTIVITY CONCENTRATES MORE IN THE THYROID THAN IN ANY OTHER TISSUE, WITH THYROID:PLASMA RATIO REACHING 62.5 AFTER 4 DAYS' CONTINUOUS DOSING.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 164
COMPLETE ABSORPTION...FROM ORAL DOSES...DEMONSTRATED IN RATS. ...IS NEGLIGIBLY BOUND TO PLASMA PROTEINS &...EXHIBITS SINGLE-COMPARTMENT KINETICS EVEN AFTER IV DOSES. ...ATTRIBUTED TO FASTER TISSUE PENETRATION BY METHIMAZOLE DUE TO ITS HIGHER LIPID:WATER PARTITION COEFFICIENT.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 164
BILIARY EXCRETION OF RADIOACTIVITY FROM...(35)S-METHIMAZOLE...AMOUNTED TO ONLY 21%...OF IV DOSES. BILIARY RADIOACTIVITY WAS ALMOST ENTIRELY DUE TO METABOLITES...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 163
For more Absorption, Distribution and Excretion (Complete) data for METHIMAZOLE (9 total), please visit the HSDB record page.
Methimazole is rapidly and extensively metabolized by the liver, mainly via the CYP450 and FMO enzyme systems. Several metabolites have been identified, though the specific enzyme isoforms responsible for their formation are not entirely clear. One of the first methimazole metabolites identified, 3-methyl-2-thiohydantoin, may contribute to antithyroid activity - its antithyroid activity has been demonstrated in rats and may explain the prolonged duration of iodination inhibition following administration despite methimazole's relatively short half-life. A number of metabolites have been investigated as being the culprits behind methimazole-induced hepatotoxicity. Both glyoxal and N-methylthiourea have established cytotoxicity and are known metabolic products of methimazole's dihydrodiol intermediate. Sulfenic and sulfinic acid derivatives of methimazole are thought to be the ultimate toxicants responsible for hepatotoxicity, though their origin is unclear - they may arise from direct oxidation of methimazole via FMO, or from oxidation of N-methylthiourea further downstream in the metabolic process.
ADMIN...TO SPRAGUE-DAWLEY RATS...UP TO 21% OF DOSE...EXCRETED UNCHANGED IN 24-HR URINE, IN WHICH MAJOR METABOLITE WAS...GLUCURONIDE (36-48%); REMAINING URINARY METABOLITE HAS NOT BEEN IDENTIFIED.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 307
INCUBATION OF METHIMAZOLE WITH RAT HEPATIC MICROSOMES LED TO FORMATION OF 3-METHYL-2-THIOHYDANTOIN & N-METHYLIMIDAZOLE.
LEE PW, NEAL RA; DRUG METAB DISPOS 6(5) 591 (1978)
Following a single intravenous bolus injection of 10mg of methimazole, the t1/2 of the distribution phase was 0.17 hours and the t1/2 of the elimination phase was 5.3 hours. Methimazole's primary active metabolite, 3-methyl-2-thiohydantoin, has a half-life approximately 3 times longer than its parent drug. Renal impairment does not appear to alter the half-life of methimazole, but patients with hepatic impairment showed an increase in half-life roughly proportional to the severity of their impairment - moderate insufficiency resulted in a elimination t1/2 of 7.1 hours, while severe insufficiency resulted in an elimination t1/2 of 22.1 hours. There does not appear to be any significant differences in half-life based on thyroid status (i.e. no difference between euthyroid and hyperthyroid patients).
Plasma half-life is 3-5 hr
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997.
The elimination half-life of methimazole reportedly ranges from about 5-13 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 2650
T/2...IN PLASMA...FOR METHIMAZOLE IS ABOUT 4-6 HR. ...
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1399
Methimazole's primary mechanism of action appears to be interference in an early step in thyroid hormone synthesis involving thyroid peroxidase (TPO), however the exact method through which methimazole inhibits this step is unclear. TPO, along with hydrogen peroxide, normally catalyzes the conversion of iodide to iodine and then further catalyzes the incorporation of this iodine onto the 3 and/or 5 positions of the phenol rings of tyrosine residues in thyroglobulin. These thyroglobulin molecules then degrade within thyroid follicular cells to form either thyroxine (T4) or tri-iodothyronine (T3), which are the main hormones produced by the thyroid gland. Methimazole may directly inhibit TPO, but has been shown in vivo to instead act as a competitive substrate for TPO, thus becoming iodinated itself and interfering with the iodination of thyroglobulin. Another proposed theory is that methimazoles sulfur moiety may interact directly with the iron atom at the centre of TPOs heme molecule, thus inhibiting its ability to iodinate tyrosine residues. Other proposed mechanisms with weaker evidence include methimazole binding directly to thyroglobulin or direct inhibition of thyroglobulin itself.
ANTITHYROID DRUGS INHIBIT FORMATION OF THYROID HORMONE LARGELY BY INTERFERING WITH INCORPORATION OF IODINE INTO ORGANIC FORM. ...IMPLIES THAT THEY INTERFERE WITH OXIDATION OF IODIDE ION.../WHICH/ IS PROBABLY BROUGHT ABOUT BY PEROXIDASE. /ANTITHYROID DRUGS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1411
ANTITHYROID DRUGS INHIBIT THE FORMATION OF THYROID HORMONES BY ITERFERING WITH THE INCORPORATION OF IODINE INTO TYROSYL RESIDUES OF THYROGLOBULIN; THEY ALSO INHIBIT THE COUPLING OF THESE IODOTYROSYL RESIDUES TO FORM IODOTHYRONINES.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1398
Methimazole inhibits the synthesis of thyroid hormones by interfering with the incorporation of iodine into tyrosyl residues of thyroglobulin; the drug also inhibits the coupling of these iodotyrosyl residues to form iodothyronine. Although the exact mechanism(s) has not been fully elucidated, methimazole may interfere with the oxidation of iodide ion and iodotyrosyl groups. Based on limited evidence, it appears that the coupling reaction is more sensitive to antithyroid agents than the iodination reaction. Methimazole does not inhibit the action of thyroid hormones already formed and present in the thyroid gland or circulation nor does the drug interfere with the effectiveness of exogenously administered thyroid hormones.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 2650