
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
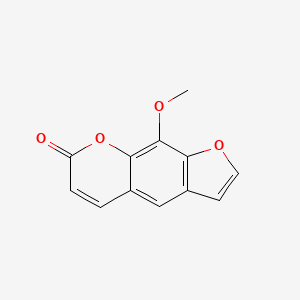

1. 8 Methoxypsoralen
2. 8 Mop
3. 8-methoxypsoralen
4. 8-mop
5. 8mop
6. Ammoidin
7. Deltasoralen
8. Dermox
9. Geroxalen
10. Mladinine
11. Meladinina
12. Meladinine
13. Meloxine
14. Methoxa Dome
15. Methoxa-dome
16. Oxsoralen
17. Oxsoralen Ultra
18. Oxsoralen-ultra
19. Puvalen
20. Ultramop
21. Xanthotoxin
1. 8-methoxypsoralen
2. 298-81-7
3. Xanthotoxin
4. Ammoidin
5. 8-mop
6. Meladinine
7. Oxsoralen
8. Oxypsoralen
9. Puvalen
10. 9-methoxy-7h-furo[3,2-g]chromen-7-one
11. Xanthotoxine
12. Meloxine
13. Meladinin
14. Uvadex
15. Meladoxen
16. Ammodin
17. Methoxa-dome
18. 8-methoxypsoralene
19. Oxsoralen-ultra
20. New-meladinin
21. Geroxalen
22. Methoxalen
23. Puvamet
24. Oxsoralen Lotion
25. 9-methoxypsoralen
26. Zanthotoxin
27. 9-methoxyfuro[3,2-g]chromen-7-one
28. Psoralon-mop
29. 8-mp
30. 8-methoxyfuranocoumarin
31. 9-methoxy-7h-furo[3,2-g][1]benzopyran-7-one
32. O-methylxanthotoxol
33. Vitpso
34. 8-methoxy-2',3',6,7-furocoumarin
35. Nci-c55903
36. Methoxy-8-psoralen
37. 7h-furo[3,2-g][1]benzopyran-7-one, 9-methoxy-
38. 8-methoxy-4',5',6,7-furocoumarin
39. Metoxalen
40. 8-methoxsalen
41. 6-hydroxy-7-methoxy-5-benzofuranacrylic Acid Delta-lactone
42. 8-methoxy-[furano-3'.2':6.7-coumarin]
43. Nsc45923
44. Nsc 45923
45. 8-methoxy-4',5':6,7-furocoumarin
46. 9-methoxy-7h-furo(3,2-g)(1)benzopyran-7-one
47. 7h-furo(3,2-g)(1)benzopyran-7-one, 9-methoxy-
48. 8-methoxy-(furano-3'.2':6.7-coumarin)
49. Methoxsalen (oxsoralen)
50. Nsc-45923
51. 9-methoxyfuro(3,2-g)chromen-7-one
52. Chembl416
53. Oxsoralen Ultra
54. U4vj29l7bq
55. 8-methoxy-6,7-furanocoumarin
56. Chebi:18358
57. 9-(methyloxy)-7h-furo[3,2-g]chromen-7-one
58. Proralone-mop
59. Methoxsalen-d3
60. 9-methoxy-2h-furo[3,2-g]chromen-2-one
61. Sm-88 Component Methoxsalen
62. Meladinin (van)
63. Ncgc00016418-07
64. Cas-298-81-7
65. Methoxaten
66. Oxoralen
67. Dsstox_cid_830
68. Ultra Mop
69. Dsstox_rid_75816
70. Dsstox_gsid_20830
71. Deltapsoralen
72. Dltasoralen
73. Methoxsalene
74. Metoxaleno
75. Methoxsalen, 8-
76. Smr000071170
77. Ccris 2083
78. 1246819-63-5
79. Hsdb 2505
80. 9-methoxyfuro[3,2-g][1]benzopyran-7-one
81. 8-methoxypsoralen With Ultraviolet A Therapy
82. Oxsoralen (tn)
83. Sr-01000629727
84. Einecs 206-066-9
85. Mfcd00005009
86. Methoxsalen Plus Ultraviolet Radiation
87. Unii-u4vj29l7bq
88. Brn 0196453
89. Methoxsalen [usp:ban:jan]
90. 9-methoxy-7h-furo(3,2-g)benzopyran-7-one
91. 5-benzofuranacrylic Acid, 6-hydroxy-7-methoxy-, Delta-lactone
92. 8-methoxy Psoralen
93. Prestwick_661
94. 9-methoxyfurano[3,2-g]chromen-2-one
95. Spectrum_001023
96. St041029
97. 5-demethoxyisoimpinellin
98. 8-methoxy(furano-3'.2':6.7-coumarin)
99. Methoxsalen [mi]
100. Prestwick0_000479
101. Prestwick1_000479
102. Prestwick2_000479
103. Prestwick3_000479
104. Spectrum2_001052
105. Spectrum3_000499
106. Spectrum4_000052
107. Spectrum5_001891
108. Methoxsalen [jan]
109. Uvadex (tn)
110. X0009
111. Methoxsalen [hsdb]
112. Methoxsalen [iarc]
113. Methoxsalen (jp17/usp)
114. Methoxsalen [vandf]
115. 8-mop;xanthotoxin;ammoidin
116. 7h-furo[3, 9-methoxy-
117. Methoxsalen [mart.]
118. Oprea1_166319
119. Schembl19168
120. Bspbio_000618
121. Bspbio_001997
122. Kbiogr_000543
123. Kbioss_001503
124. Methoxsalen [usp-rs]
125. Methoxsalen [who-dd]
126. 5-19-06-00015 (beilstein Handbook Reference)
127. 8mo
128. Mls000062633
129. Mls002303011
130. Divk1c_000763
131. Spectrum1500400
132. Spbio_001004
133. Spbio_002557
134. Bpbio1_000680
135. Megxp0_000095
136. Xanthotoxin, Analytical Standard
137. Dtxsid8020830
138. Methoxsalen (8-methoxypsoralen)
139. Acon1_000174
140. Hms502g05
141. Kbio1_000763
142. Kbio2_001503
143. Kbio2_004071
144. Kbio2_006639
145. Kbio3_001497
146. 8-methoxy Psoralen-[13c,d3]
147. Methoxsalen [orange Book]
148. 8-methoxy-2',6,7-furocoumarin
149. 8-methoxy-4',6,7-furocoumarin
150. Ninds_000763
151. Hms1569o20
152. Hms1920n05
153. Hms2091d20
154. Hms2096o20
155. Hms2269p03
156. Hms3259l13
157. Hms3655b05
158. Hms3884k16
159. Pharmakon1600-01500400
160. Methoxsalen [usp Monograph]
161. 5-benzofuranacrylic Acid, 6-hydroxy-7-methoxy-, .delta.-lactone
162. Bcp28212
163. Zinc2548959
164. Tox21_110432
165. Tox21_201767
166. Tox21_302816
167. 8-methoxypsoralen;xanthotoxin;8-mop
168. Bdbm50041234
169. Ccg-36366
170. Nsc757114
171. S1952
172. Stk735539
173. Wln: T C566 Do Lvoj Bo1
174. 8-methoxypsoralen, Analytical Standard
175. Akos000277012
176. Tox21_110432_1
177. 9-methoxy-furo[3,2-g]chromen-7-one
178. Ac-4259
179. Am84906
180. Cs-1983
181. Db00553
182. Ds-5159
183. Methoxsalen 100 Microg/ml In Methanol
184. Nc00652
185. Nsc-757114
186. Sdccgmls-0042377.p002
187. Idi1_000763
188. 8-methoxy-2'',3'',6,7-furocoumarin
189. 8-methoxy-4'',5'':6,7-furocoumarin
190. Ncgc00016418-01
191. Ncgc00016418-02
192. Ncgc00016418-03
193. Ncgc00016418-04
194. Ncgc00016418-05
195. Ncgc00016418-06
196. Ncgc00016418-08
197. Ncgc00016418-09
198. Ncgc00016418-10
199. Ncgc00016418-11
200. Ncgc00016418-12
201. Ncgc00016418-14
202. Ncgc00016418-15
203. Ncgc00060938-02
204. Ncgc00060938-03
205. Ncgc00060938-04
206. Ncgc00060938-05
207. Ncgc00060938-06
208. Ncgc00178871-01
209. Ncgc00178871-02
210. Ncgc00178871-03
211. Ncgc00256435-01
212. Ncgc00259316-01
213. Hy-30151
214. Nci60_004085
215. Sbi-0051443.p003
216. Ft-0602101
217. Ft-0671145
218. N1305
219. Sw167762-4
220. 8-methoxy-[furano-3''.2'':6.7-coumarin]
221. 9-methoxy-7h-furo[3,2-g]chromen-7-one #
222. En300-52504
223. C01864
224. D00139
225. Ab00052042-14
226. Ab00052042-15
227. Ab00052042_16
228. Ab00052042_17
229. 298m817
230. 9-methoxy-7h-furo[3,2- G][1]benzopyran-7-one
231. A820092
232. Q408570
233. Q-100381
234. Sr-01000629727-2
235. Sr-01000629727-4
236. Brd-k63430059-001-03-2
237. Brd-k63430059-001-06-5
238. Brd-k63430059-001-09-9
239. Z1258578369
240. Methoxsalen, United States Pharmacopeia (usp) Reference Standard
241. 12692-94-3
| Molecular Weight | 216.19 g/mol |
|---|---|
| Molecular Formula | C12H8O4 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 216.04225873 g/mol |
| Monoisotopic Mass | 216.04225873 g/mol |
| Topological Polar Surface Area | 48.7 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 325 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | 8-mop |
| PubMed Health | Methoxsalen |
| Drug Classes | Antipsoriatic, Hypopigmentation Agent |
| Drug Label | 8-MOP (Methoxsalen, 8-Methoxypsoralen) Capsules, 10mg. Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant and in the roots of Heracleum Candicans. It belongs to a group of compounds kn... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 2 of 10 | |
|---|---|
| Drug Name | Methoxsalen |
| PubMed Health | Methoxsalen |
| Drug Classes | Antipsoriatic, Hypopigmentation Agent |
| Drug Label | 8-MOP (Methoxsalen, 8-Methoxypsoralen) Capsules, 10mg. Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant and in the roots of Heracleum Candicans. It belongs to a group of compounds kn... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Strides Pharma |
| 3 of 10 | |
|---|---|
| Drug Name | Oxsoralen |
| PubMed Health | Methoxsalen (By mouth) |
| Drug Classes | Antipsoriatic |
| Drug Label | Oxsoralen-Ultra (methoxsalen capsules, USP) contains 10 mg. methoxsalen (8-methoxsalen). Methoxsalen occurs as white to pale yellow crystals and can be obtained naturally from seeds of Ammi majus and roots of Heracleum Candicans or through synthesis.... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Lotion |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 4 of 10 | |
|---|---|
| Drug Name | Oxsoralen-ultra |
| PubMed Health | Methoxsalen (Injection) |
| Drug Classes | Antipsoriatic |
| Drug Label | Oxsoralen-Ultra (methoxsalen capsules, USP) contains 10 mg. methoxsalen (8-methoxsalen). Methoxsalen occurs as white to pale yellow crystals and can be obtained naturally from seeds of Ammi majus and roots of Heracleum Candicans or through synthesis.... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Dow Pharm |
| 5 of 10 | |
|---|---|
| Drug Name | Uvadex |
| Drug Label | Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant. It belongs to a group of compounds known as psoralens or furocoumarins. The chemical name of methoxsalen is 9-methoxy-7H-furo[3,2-g]... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/ml |
| Market Status | Prescription |
| Company | Therakos |
| 6 of 10 | |
|---|---|
| Drug Name | 8-mop |
| PubMed Health | Methoxsalen |
| Drug Classes | Antipsoriatic, Hypopigmentation Agent |
| Drug Label | 8-MOP (Methoxsalen, 8-Methoxypsoralen) Capsules, 10mg. Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant and in the roots of Heracleum Candicans. It belongs to a group of compounds kn... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 7 of 10 | |
|---|---|
| Drug Name | Methoxsalen |
| PubMed Health | Methoxsalen |
| Drug Classes | Antipsoriatic, Hypopigmentation Agent |
| Drug Label | 8-MOP (Methoxsalen, 8-Methoxypsoralen) Capsules, 10mg. Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant and in the roots of Heracleum Candicans. It belongs to a group of compounds kn... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Strides Pharma |
| 8 of 10 | |
|---|---|
| Drug Name | Oxsoralen |
| PubMed Health | Methoxsalen (By mouth) |
| Drug Classes | Antipsoriatic |
| Drug Label | Oxsoralen-Ultra (methoxsalen capsules, USP) contains 10 mg. methoxsalen (8-methoxsalen). Methoxsalen occurs as white to pale yellow crystals and can be obtained naturally from seeds of Ammi majus and roots of Heracleum Candicans or through synthesis.... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Lotion |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 9 of 10 | |
|---|---|
| Drug Name | Oxsoralen-ultra |
| PubMed Health | Methoxsalen (Injection) |
| Drug Classes | Antipsoriatic |
| Drug Label | Oxsoralen-Ultra (methoxsalen capsules, USP) contains 10 mg. methoxsalen (8-methoxsalen). Methoxsalen occurs as white to pale yellow crystals and can be obtained naturally from seeds of Ammi majus and roots of Heracleum Candicans or through synthesis.... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg |
| Market Status | Prescription |
| Company | Dow Pharm |
| 10 of 10 | |
|---|---|
| Drug Name | Uvadex |
| Drug Label | Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant. It belongs to a group of compounds known as psoralens or furocoumarins. The chemical name of methoxsalen is 9-methoxy-7H-furo[3,2-g]... |
| Active Ingredient | Methoxsalen |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/ml |
| Market Status | Prescription |
| Company | Therakos |
Cross-Linking Reagents; Photosensitizing Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2019)
Photochemotherapy (methoxsalen with long wave UVA radiation) is indicated for the symptomatic control of severe, recalcitrant, disabling psoriasis not adequately responsive to other forms of therapy and when the diagnosis has been supported by biopsy. Photochemotherapy is intended to be administered only in conjunction with a schedule of controlled doses of long wave ultraviolet radiation. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for METHOXSALEN- methoxsalen capsule, liquid filled (May 2015).
Methoxsalen is used orally (as conventional capsules) or topically in conjunction with controlled exposure to long wavelength ultraviolet radiation (UVA) or sunlight to repigment vitiliginous skin in patients with idiopathic vitiligo. The liquid-filled capsules currently are not approved by the US Food and Drug Administration for this use. Clinical response to methoxsalen is erratic and unpredictable and is cosmetically acceptable in only a small percentage of patients with vitiligo. Complete cures following psoralen therapy are infrequent; only about one-third of patients with vitiligo have an appreciable amount of pigmentation restored. In one study of 20 patients treated with topical methoxsalen and blacklight, complete repigmentation occurred in only 3 patients. In one study using UVA light and oral methoxsalen or oral trioxsalen for 12-14 months, 73% of vitiligo patients had some pigmentation restored and, in 23% of patients, pigmentation improved by about 73%. Repigmentation varies among patients in completeness, time of onset, and duration. Methoxsalen-induced repigmentation occurs more rapidly on fleshy areas such as the face, abdomen, and buttocks than on bony areas such as the dorsa of the hands and feet. To retain new pigment, periodic treatment with the drug and some form of UVA light is often required; however, in one study, 90% or more of the new pigment established during oral methoxsalen and conventional UV light therapy remained in 85% of patients 8-14 years after methoxsalen treatment was discontinued.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Oral methoxsalen is used in conjunction with photopheresis with the UVAR instrument for the palliative treatment of the skin manifestations of cutaneous T-cell lymphoma (CTCL; e.g., mycosis fungoides, Sezary syndrome). Limited evidence indicates that photopheresis therapy (e.g., administered once daily for 2 consecutive days per month) can produce reductions in the size and/or severity of skin lesions without serious toxicity; sustained responses (2 years or longer) have occurred in some patients. For detailed information on the use of oral methoxsalen in conjunction with photopheresis in patients with cutaneous T-cell lymphoma, clinicians should consult the manufacturer's labeling for methoxsalen and the UVAR instrument and other specialized references and published protocols.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
For more Therapeutic Uses (Complete) data for 8-Methoxypsoralen (6 total), please visit the HSDB record page.
/BOXED WARNING/ Methoxsalen with UV radiation should be used only by physicians who have special competence in the diagnosis and treatment of psoriasis and who have special training and experience in photochemotherapy. The use of Psoralen and ultraviolent radiation therapy should be under constant supervision of such a physician. For the treatment of patients with psoriasis, photochemotherapy should be restricted to patients with severe, recalcitrant, disabling psoriasis which is not adequately responsive to other forms of therapy, and only when the diagnosis is certain. Because of the possibilities of ocular damage, aging of the skin, and skin cancer (including melanoma), the patient should be fully informed by the physician of the risks inherent in this therapy.
US Natl Inst Health; DailyMed. Current Medication Information for METHOXSALEN- methoxsalen capsule, liquid filled (May 2015). Available from, as of December 26, 2018: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a4c61db9-b72d-4b67-b69b-a97257b614ad
/BOXED WARNING/ Methoxsalen Capsules, USP (Soft Gelatin Capsules) should not be used interchangeably with regular methoxsalen capsules or methoxsalen hard gelatin capsules. This new dosage form of methoxsalen exhibits significantly greater bioavailability and earlier photosensitization onset time than previous methoxsalen dosage forms. Patient should be treated in accordance with the dosimetry specifically recommended for this product. The minimum phototoxic dose (MPD) and phototoxic peak time after drug administration prior to onset of photochemotherapy with this dosage form should be determined.
US Natl Inst Health; DailyMed. Current Medication Information for METHOXSALEN- methoxsalen capsule, liquid filled (May 2015). Available from, as of December 26, 2018: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a4c61db9-b72d-4b67-b69b-a97257b614ad
/Methoxsalen is contraindicated in:/ patients exhibiting idiosyncratic reactions to psoralen compounds; patients possessing a specific history of light sensitive disease states should not initiate methoxsalen therapy. Diseases associated with photosensitivity include lupus erythematosus, porphyria cutanea tarda, erythropoietic protoporphyria, variegate porphyria, xeroderma pigmentosum, and albinism; patients exhibiting melanoma or possessing a history of melanoma; patients exhibiting invasive squamous cell carcinomas; patients with aphakia, because of the significantly increased risk of retinal damage due to the absence of lenses.
US Natl Inst Health; DailyMed. Current Medication Information for METHOXSALEN- methoxsalen capsule, liquid filled (May 2015). Available from, as of December 26, 2018: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a4c61db9-b72d-4b67-b69b-a97257b614ad
Phototoxic reactions including severe edema and erythema, and painful blistering, burning, and peeling of skin may occur with methoxsalen and conventional UV light. In addition, PUVA therapy has produced severe burns requiring hospitalization, and marked hyperpigmentation and aging of skin. When peeling or blistering occurs, the skin becomes more sensitive to UV light. Phototoxic reactions to methoxsalen occur most commonly when the skin is overexposed to UV light or when dosage is excessive. Severe burns may occur if treated skin is accidentally exposed to additional UV light. Some reports indicate that the incidence of psoralen-induced phototoxicity may be slightly reduced by concurrent application of benzophenone sunscreens.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
For more Drug Warnings (Complete) data for 8-Methoxypsoralen (27 total), please visit the HSDB record page.
For the treatment of psoriasis and vitiligo
FDA Label
Methoxsalen selectively inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of Methoxsalen-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed.
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
Cross-Linking Reagents
Reagents with two reactive groups, usually at opposite ends of the molecule, that are capable of reacting with and thereby forming bridges between side chains of amino acids in proteins; the locations of naturally reactive areas within proteins can thereby be identified; may also be used for other macromolecules, like glycoproteins, nucleic acids, or other. (See all compounds classified as Cross-Linking Reagents.)
D - Dermatologicals
D05 - Antipsoriatics
D05A - Antipsoriatics for topical use
D05AD - Psoralens for topical use
D05AD02 - Methoxsalen
D - Dermatologicals
D05 - Antipsoriatics
D05B - Antipsoriatics for systemic use
D05BA - Psoralens for systemic use
D05BA02 - Methoxsalen
Route of Elimination
In both mice and man, methoxsalen is rapidly metabolized. Approximately 95% of the drug is excreted as a series of metabolites in the urine within 24 hours (Pathak et al. 1977).
After oral admin of (3)H 8-methoxypsoralen to rats, it was absorbed rapidly and max blood level was observed at 10 min. Moderate radioactivity was found in liver and kidneys at 0.5-4 hr, and low levels in other tissues. ... 62.8% of radioactivity was excreted in urine and 20.4% in feces within 24 hr and 65.1% and 21.9% during 6 days, respectively. In bile, 30.0% was also recovered within 24 hr; this passed through enterohepatic circulation.
NOZU T ET AL; OYO YAKURI 18 (3): 489 (1979)
Improvement in effective bioavailability of methoxsalen was achieved when it was administered to rats and dogs in solution as compared to suspension. Much earlier and higher peak levels were observed for the solution in both animals.
PMID:438967 KREUTER J, HIGUCHI T; J PHARM SCI; 68 (4): 451 (1979)
Max serum concentration /in patients/ occurred between 0.5 and 2 hr after oral administration of 0.6 mg/kg methoxsalen. There was significant negative correlation between logarithm of serum concentration and minimum phototoxic dose. Hence degree of photosensitivity appears to be related to serum level of methoxsalen.
PMID:428192 SWANBECK G ET AL; CLIN PHARMACOL THER 25 (4): 478 (1979)
Single iv doses of 5 mg/kg body weight (14)C methoxsalen to dogs disappeared rapidly from plasma, although small levels of radioactivity persisted for 5 weeks after administration. Evidence suggested that the persistent plasma radioactivity was due to a metabolite bound to plasma protein. Elimination occurred in both urine and bile; 45% of the dose appeared in the urine and 40% in the feces within 72 hrs of administration.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V24 109 (1980)
For more Absorption, Distribution and Excretion (Complete) data for 8-Methoxypsoralen (8 total), please visit the HSDB record page.
After oral administration of 8-methoxypsoralen to rats, metabolites in urine were; 8-hydroxypsoralen, 5-hydroxy-8-methoxypsoralen, 5,8-dioxopsoralen, 5,8-dihydroxypsoralen, 4,6,7-trihydroxy-5-coumaranoyl-beta-acrylic acid, 4,6-dihydroxy-7-methoxy-5-coumaranoyl-beta-acrylic acid.
NOZU T ET AL; OYO YAKURI 18 (3): 497 (1979)
Although the exact metabolic fate of methoxsalen has not been fully established, the drug is rapidly and apparently almost completely metabolized. Methoxsalen is demethylated to 8-hydroxypsoralen (8-HOP), and methoxsalen and 8-HOP are conjugated with glucuronic acid and sulfate; other unidentified metabolites have also been detected. Methoxsalen and 8-hydroxypsoralen and their conjugates are excreted in urine. Following oral administration of methoxsalen, 80-90% of the drug is excreted in urine within 8 hours as hydroxylated, glucuronide, and sulfate metabolites; less than 0.1% of a dose is excreted in urine as unchanged drug. About 95% of the drug is excreted in urine within 24 hours as metabolites.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Methoxsalen is extensively metabolized, and less than 2% of the drug is excreted unchanged in the urine. Four urinary metabolites were isolated; 3 of them resulted from opening of the furan ring: these are 7-hydroxy-8-methoxy-2-oxo-2H-1-benzopyran-6-acetic acid, alpha,7-dihydroxy-8-methoxy-2-oxo-2H-1-benzopyran-6-acetic acid, and an unknown conjugate of the former at the 7-hydroxy position. The fourth metabolite, formed by opening of the pyrone ring, is an unknown conjugate of (Z)-3-(6-hydroxy-7-methoxybenzofuran-5-yl)-2-propenoic acid.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V24 109 (1980)
Methoxsalen has known human metabolites that include 9-Methoxy-5,7,11-trioxatetracyclo[8.4.0.03,8.04,6]tetradeca-1,3(8),9,13-tetraen-12-one.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Approximately 2 hours
The elimination half-life of methoxsalen is reportedly about 0.75-2.4 hours.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
After activation it binds preferentially to the guanine and cytosine moieties of DNA, leading to cross-linking of DNA, thus inhibiting DNA synthesis and function.
Methoxsalen, when activated by long wavelength UV light in the range of 320-400 nm, is strongly erythemogenic, melanogenic, and cytotoxic in the epidermis; the maximal erythemogenic activity occurs in the range of 330-360 nm. The mechanisms of action of methoxsalen in inducing repigmentation of vitiliginous skin have not been established. Repigmentation depends on the presence of functioning melanocytes and UV light. Methoxsalen may activate the few functional and dihydroxyphenylalanine-positive melanocytes present in vitiliginous skin. An increase in the activity of tyrosinase, the enzyme that catalyzes the conversion of tyrosine to dihydroxyphenylalanine (a precursor of melanin), has been shown in melanin-producing cells exposed in vitro to trioxsalen and UVA light. In addition, binding of photoactivated psoralens (in triplet states) to pyrimidine bases of nucleic acids, with subsequent inhibitions of DNA synthesis, cell division, and epidermal turnover, has been demonstrated. Following photoactivation, methoxsalen forms covalent bonds with DNA to produce monofunctional (addition to a single strand of DNA) and bifunctional adducts (crosslinking to both strands of DNA). Reactions with other proteins also occur. Psoralens may also increase melanin formation by producing an inflammatory reaction in the skin. Other mechanisms of increased pigmentation may include an increase in the number of functional melanocytes (and possibly activation of dormant melanocytes); enhancement of melanin granule synthesis; stimulation of the movement of melanocytes up hair follicles resulting in melanocytic repopulation of the epidermis; and/or hypertrophy of melanocytes and increased arborization of their dendrites.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Since psoriasis is a hyperproliferative disorder and other agents effective in the treatment of psoriasis are known to inhibit DNA synthesis, the therapeutic effect of methoxsalen in the treatment of psoriasis probably involves binding to DNA and inhibition of DNA synthesis resulting in decreased cell proliferation; other vascular, leukocyte, or cell regulatory mechanisms may also be involved.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Methoxsalen readily absorbs ultraviolet light, particularly UVA wavelengths (320 to 400 nm). As a photosensitizing agent, it can produce phototoxic erythema (a reaction similar to sunburn) when skin to which it has been applied receives excess exposure to UVA. Chronic reactions may result in hyperpigmentation and skin thickening. UVA causes a photochemical reaction that results in formation of adducts between methoxsalen and the pyrimidine bases of DNA.
DHHS/National Toxicology Program; Report on Carcinogens, Fourteenth Edition: Methoxsalen with Ultraviolet A Therapy; Available from, as of December 27, 2018: https://ntp.niehs.nih.gov/go/roc

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
54
PharmaCompass offers a list of Methoxsalen API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methoxsalen manufacturer or Methoxsalen supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methoxsalen manufacturer or Methoxsalen supplier.
PharmaCompass also assists you with knowing the Methoxsalen API Price utilized in the formulation of products. Methoxsalen API Price is not always fixed or binding as the Methoxsalen Price is obtained through a variety of data sources. The Methoxsalen Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Methoxsalen manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Methoxsalen, including repackagers and relabelers. The FDA regulates Methoxsalen manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Methoxsalen API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Methoxsalen manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Methoxsalen supplier is an individual or a company that provides Methoxsalen active pharmaceutical ingredient (API) or Methoxsalen finished formulations upon request. The Methoxsalen suppliers may include Methoxsalen API manufacturers, exporters, distributors and traders.
click here to find a list of Methoxsalen suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Methoxsalen DMF (Drug Master File) is a document detailing the whole manufacturing process of Methoxsalen active pharmaceutical ingredient (API) in detail. Different forms of Methoxsalen DMFs exist exist since differing nations have different regulations, such as Methoxsalen USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Methoxsalen DMF submitted to regulatory agencies in the US is known as a USDMF. Methoxsalen USDMF includes data on Methoxsalen's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Methoxsalen USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Methoxsalen suppliers with USDMF on PharmaCompass.
A Methoxsalen written confirmation (Methoxsalen WC) is an official document issued by a regulatory agency to a Methoxsalen manufacturer, verifying that the manufacturing facility of a Methoxsalen active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Methoxsalen APIs or Methoxsalen finished pharmaceutical products to another nation, regulatory agencies frequently require a Methoxsalen WC (written confirmation) as part of the regulatory process.
click here to find a list of Methoxsalen suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Methoxsalen as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Methoxsalen API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Methoxsalen as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Methoxsalen and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Methoxsalen NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Methoxsalen suppliers with NDC on PharmaCompass.
Methoxsalen Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Methoxsalen GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Methoxsalen GMP manufacturer or Methoxsalen GMP API supplier for your needs.
A Methoxsalen CoA (Certificate of Analysis) is a formal document that attests to Methoxsalen's compliance with Methoxsalen specifications and serves as a tool for batch-level quality control.
Methoxsalen CoA mostly includes findings from lab analyses of a specific batch. For each Methoxsalen CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Methoxsalen may be tested according to a variety of international standards, such as European Pharmacopoeia (Methoxsalen EP), Methoxsalen JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Methoxsalen USP).
