
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
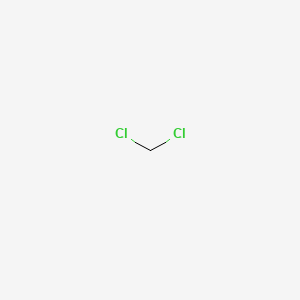

1. Bichloride, Methylene
2. Chloride, Methylene
3. Dichloride, Methylene
4. Dichloromethane
5. Methylene Bichloride
6. Methylene Dichloride
7. Solaesthin
1. Dichloromethane
2. 75-09-2
3. Methylene Dichloride
4. Methane, Dichloro-
5. Methylene Bichloride
6. Methane Dichloride
7. Solaesthin
8. Solmethine
9. Freon 30
10. Narkotil
11. Aerothene Mm
12. Metylenu Chlorek
13. Chlorure De Methylene
14. Dichlormethan
15. Metaclen
16. Soleana Vda
17. Ch2cl2
18. Khladon 30
19. Dichloro-methane
20. F 30 (chlorocarbon)
21. Rcra Waste Number U080
22. Nci-c50102
23. R 30
24. Dichlormethane
25. Methoklone
26. Hcc 30
27. Salesthin
28. Dichloro Methane
29. Chlorodorm D
30. Nsc 406122
31. Un 1593
32. F 30
33. Methylene Chloride [nf]
34. Dichloromethane, Hplc Grade
35. 588x2yuy0a
36. Chebi:15767
37. Mfcd00000881
38. Nsc-406122
39. Methylene Chloride (nf)
40. Methylenum Chloratum
41. R30 (refrigerant)
42. Caswell No. 568
43. Metylenu Chlorek [polish]
44. Hsdb 66
45. Ccris 392
46. Chloride, Methylene
47. Chlorure De Methylene [french]
48. Bichloride, Methylene
49. Dichloride, Methylene
50. Einecs 200-838-9
51. Un1593
52. Dichloromethane, Nf
53. Rcra Waste No. U080
54. Dichloromethane, Acs
55. Epa Pesticide Chemical Code 042004
56. Brn 1730800
57. Dichioromethane
58. Dichlormetane
59. Dichloromeihane
60. Dichlorometan
61. Dichlorometane
62. Dichloromethan
63. Dichoromethane
64. Dicloromethane
65. Methylenchoride
66. Metylenchloride
67. Unii-588x2yuy0a
68. Methylenchlorid
69. Aerothene
70. Driverit
71. Nevolin
72. Dichlor-methane
73. Dichlorometliane
74. Dichlorornethane
75. Dicliloromethane
76. Methylenchloride
77. Methylenechlorid
78. Methyienechlorid
79. Di-chloromethane
80. Dichloromethane-
81. Methlyenechloride
82. Methylenechloride
83. Ai3-01773
84. Dichloromethane, Suitable For 5000 Per Jis, For Residue Analysis
85. Methlene Chloride
86. Methyene Chloride
87. Methylen Chloride
88. Methylene Chlorie
89. Methylene Cloride
90. Metylene Chloride
91. Methylene Choride
92. Mehtylene Chloride
93. Methlyene Chloride
94. Methylene,chloride
95. Methylene-chloride
96. Dichloro -methane
97. Dichloro- Methane
98. Methylenedichloride
99. Distillex Ds3
100. Dichloromethane, Acs Reagent, >=99.5%, Contains 40-150 Ppm Amylene As Stabilizer
101. M-clean D
102. Methyl Ene Chloride
103. Dichloromethane (methylene Chloride)
104. Mecl2
105. Dcm,sp Grade
106. Methylene Di Chloride
107. N,n-methylenechloride
108. Dichloromethane Solution
109. Methylene Chloride Acs
110. Cl2ch2
111. H2ccl2
112. Dsstox_cid_868
113. Dichloromethane, Anhydrous
114. Dichloromethane, For Hplc
115. Ec 200-838-9
116. Ncimech_000221
117. Wln: G1g
118. Dsstox_rid_75836
119. Dsstox_gsid_20868
120. 4-01-00-00035 (beilstein Handbook Reference)
121. Dichloromethane, >=99.9%
122. Chembl45967
123. Dichloromethane [iarc]
124. Methylene Chloride (recovered)
125. Dichloromethane, Ar, >=99%
126. Dichloromethane (peptide Grade)
127. Dichloromethane [mart.]
128. Methylene Chloride [ii]
129. Methylene Chloride [mi]
130. Dtxsid0020868
131. Methylene Chloride [fcc]
132. Dtxsid60166893
133. Methylene Chloride [hsdb]
134. Dichloromethane Reagent Grade Acs
135. Dichloromethane, Lr, >=99.5%
136. Dichloromethane, Purification Grade
137. Methylene Chloride [vandf]
138. Dichloromethane, Analytical Standard
139. Dichloromethane, Environmental Grade
140. Methylene Chloride [usp-rs]
141. Tox21_202526
142. Nsc406122
143. Stl264204
144. Akos009031498
145. Dichloromethane [un1593] [poison]
146. Dichloromethane, Acs Reagent, 99.5%
147. Cas-75-09-2
148. Dichloromethane, For Hplc, >=99.7%
149. Dichloromethane Gc, For Residue Analysis
150. Dichloromethane, Spectrophotometric Grade
151. Methylene Chloride [ep Monograph]
152. Ncgc00091504-01
153. Ncgc00260075-01
154. Dichloromethane 100 Microg/ml In Methanol
155. Dichloromethane, Suitable For Pcb Analysis
156. Dichloromethane 1000 Microg/ml In Methanol
157. D0529
158. D3478
159. Ft-0624716
160. Ft-0624717
161. M0629
162. Dichloromethane, 99%, Stabilized With Ethanol
163. Dichloromethane, For Hplc, >=99.8% (gc)
164. Dichloromethane, Saj First Grade, >=99.0%
165. Dichloromethane, Selectophore(tm), >=99.5%
166. C02271
167. D02330
168. Dichloromethane, Analytical Standard, Stabilized
169. Dichloromethane, Jis Special Grade, >=99.0%
170. L023970
171. Q421748
172. Q425210
173. J-610006
174. Dichloromethane, 99%, Stab. With Ca. 50ppm Amylene
175. Methylene Chloride Hplc Grade Stabilized With Amylene
176. Dichloromethane Solution, Contains 10 % (v/v) Methanol
177. Dichloromethane, Glass Distilled Hrgc/hplc Trace Grade
178. Dichloromethane, Tlc High-purity Grade, >=99.8% (gc)
179. Dichloromethane Hplc, Uv/ir, Min. 99.9%, Isocratic Grade
180. Dichloromethane, Special, 99.9%, Contains 40-60 Ppm Amylene
181. Dichloromethane, For Hplc, >=99.8%, Contains Amylene As Stabilizer
182. Dichloromethane, Selectophore(tm), >=99.5% (gc), Inhibitor-free
183. Dichloromethane, Suitable For 300 Per Jis, For Residue Analysis
184. Dichloromethane, Technical Grade, 95%, Contains 40-60 Ppm Amylene
185. Methylene Chloride, European Pharmacopoeia (ep) Reference Standard
186. Dichloromethane, Puriss. P.a., Acs Reagent, Reag. Iso, >=99.9% (gc)
187. Dichloromethane, Uv Hplc Spectroscopic, 99.9%, Contains 40-60 Ppm Amylene
188. Dichloromethane Solution, 10 % (v/v) In Methanol, 1 % (v/v) In Ammonium Hydroxide
189. Dichloromethane Solution, Certified Reference Material, 200 Mug/ml In Methanol
190. Dichloromethane Solution, Certified Reference Material, 5000 Mug/ml In Methanol
191. Dichloromethane, Acs Reagent, >=99.5%, Contains 50 Ppm Amylene As Stabilizer
192. Dichloromethane, Anhydrous, >=99.8%, Contains 40-150 Ppm Amylene As Stabilizer
193. Dichloromethane, Anhydrous, Contains 40-150 Ppm Amylene As Stabilizer, Zero2(tm), >=99.8%
194. Dichloromethane, Biotech. Grade, 99.9%, Contains 40-150 Ppm Amylene As Stabilizer
195. Dichloromethane, Contains 40-150 Ppm Amylene As Stabilizer, Acs Reagent, >=99.5%
196. Dichloromethane, For Hplc, >=99.9%, Contains 40-150 Ppm Amylene As Stabilizer
197. Dichloromethane, Puriss., Meets Analytical Specification Of Ph.??eur., Nf, >=99% (gc)
198. Dichloromethane, Suitable For 1000 Per Jis, >=99.5%, For Residue Analysis
199. Methylene Chloride, Pharmaceutical Secondary Standard; Certified Reference Material
200. Dichloromethane, >=99.9%, Capillary Gc Grade, Suitable For Environmental Analysis, Contains Amylene As Stabilizer
201. Dichloromethane, Acs Spectrophotometric Grade, >=99.5%, Contains 50-150 Ppm Amylene As Stabilizer
202. Dichloromethane, Hplc Plus, For Hplc, Gc, And Residue Analysis, >=99.9%, Contains 50-150 Ppm Amylene As Stabilizer
203. Dichloromethane, Laboratory Reagent, >=99.9% (without Stabilizer, Gc), Contains 0.1-0.4% Ethanol As Stabilizer
204. Dichloromethane, P.a., Acs Reagent, Reag. Iso, Reag. Ph. Eur., 99.8%, Contains 40-60 Ppm Amylene
205. Dichloromethane, Puriss. P.a., Acs Reagent, Reag. Iso, Dried, >=99.8% (gc), <=0.001% Water
206. M.c
207. Residual Solvent Class 2 - Methylene Chloride, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 84.93 g/mol |
|---|---|
| Molecular Formula | CH2Cl2 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 83.9533555 g/mol |
| Monoisotopic Mass | 83.9533555 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 2.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Methylene chloride is removed from the body mainly in expired air and urine. In four human subjects exposed to methylene chloride (350 mg/cu m) for 2 hr, an average of 22.6 microg methylene chloride was excreted in the urine within 24 hr after the exposure. In seven subjects exposed to 710 mg/cu m for 2 hr, the corresponding value was 81.5 ug. These data show that the amount excreted in the urine is insignificant. Methylene chloride excretion in expired air was most evident during the first 30 min after exposure. Initial post-exposure concentrations of methylene chloride in expired breath following 2-and 4-hr exposure periods were about 71 mg/cu m and fell to about 18 mg/cu m at the end of 30 min. Small amounts of methylene chloride remained in the expired air at 2.5 hr.
International Programme on Chemical Safety/ Environmental Health Criteria 164; Methylene Chloride (Second Edition). (1996). Available from, as of July 18, 2014: https://www.inchem.org/documents/ehc/ehc/ehc164.htm
The fat content of the body was calculated in 12 healthy male subjects aged 21 to 35 years by means of hydrostatic weighing and anthropometric estimation of skeletal weight. The subjects were exposed to a concentration of 2,600 mg of methylene chloride per cubic meter of inspired air (750 ppm) for 1 hr while performing work at an intensity of 50 W on a bicycle ergometer. The uptake in the organism was measured continuously with the Douglas bag technique. The amount of methylene chloride absorbed correlated highly with degree of obesity and body weight. Needle biopsy specimens of subcutaneous adipose tissue were taken from the buttocks before exposure and 0, 1, 2, 3 and 4 hr after exposure. The mean yield of tissue from the 72 biopsies was 25 mg. The concentration of methylene chloride in the adipose tissue was determined by gas chromatography, using a headspace method. The mean concentration was 10.2 mg/kg 1 hr after exposure and 8.4 mg/kg after 4 hr. There was a wide distribution around the mean values. In the six slim subjects the concentration in the adipose tissue during the 4 hr after exposure was on an average twice that of the six more obese subjects. On the other hand, in spite of lower concentrations, the obese subjects had a greater calculated amount of methylene chloride in the total fat depots of the body. Two subjects were studied about 22 hr after exposure, the concentration in subcutaneous adipose tissue being 1.6 and 1.7 mg/kg, respectively, at that time.
PMID:594729 Engstrom J et al; Scand J Work Environ Health. 3(4):215-24 (1977) https://www.inchem.org/documents/ehc/ehc/ehc164.htm
A detailed study of the relationship between the measurements of methylene chloride in expired air or blood, carbon monoxide in expired air and CO-Hb in blood was undertaken... At the end of exposure of non-smoking, sedentary volunteers for 7.5 hr to methylene chloride vapour concentrations of 180-710 mg/cu m, the mean concentration of the solvent in alveolar air and in blood, and the percent CO-Hb saturation were measured... By 7 hr after exposure to any concentration, the expired air contained less than 3.5 mg/cu m methylene chloride; at 16 hr, only negligible levels were detected. These data suggest that, due to its rapid elimination, measurements of methylene chloride in expired air are unsuitable for use as a marker of occupational exposure.
International Programme on Chemical Safety/ Environmental Health Criteria 164; Methylene Chloride (Second Edition). (1996). Available from, as of July 18, 2014: https://www.inchem.org/documents/ehc/ehc/ehc164.htm
...the effects of exercise and cigarette smoking on the uptake, metabolism and excretion of methylene chloride /was investigated/. The effects of smoking and methylene chloride exposure on CO-Hb saturation levels were found to be additive. Exercise was found to increase the absorption of methylene chloride and CO-Hb levels. However, the effects of exercise on CO-Hb were not observed to increase with heavy workloads beyond the level achieved with moderate work-loads, suggesting a saturation of this effect...
International Programme on Chemical Safety/ Environmental Health Criteria 164; Methylene Chloride (Second Edition). (1996). Available from, as of July 18, 2014: https://www.inchem.org/documents/ehc/ehc/ehc164.htm
For more Absorption, Distribution and Excretion (Complete) data for DICHLOROMETHANE (25 total), please visit the HSDB record page.
A modified version of the original physiologically based pharmacokinetic (PBPK) model by Andersen et al. (1987) has been developed and used in conjunction with previously published human kinetic data for dichloromethane (DCM) metabolism and to assess interindividual variability in the rate of oxidative metabolism. Time-course data for 13 volunteers (10 males, 3 females) exposed to one or more concentrations of DCM (50 ppm, 100 ppm, 150 ppm, or 200 ppm) for 7.5 hr were used to optimize the maximal rate of hepatic metabolism (V(maxC)) through the cytochrome P450 pathway for each individual. DCM breath and blood concentrations were used, along with carboxyhemoglobin concentrations in blood and carbon monoxide (CO) concentrations in exhaled breath, to estimate the model parameters. Significant improvements in model fit were achieved when extrahepatic oxidative metabolism of DCM was added to the model structure. The 13 individual V(maxC) values ranged from 7.1 to 23.6 mg/hr/kg0.7 and appeared to be bimodally distributed. The distribution was not sex related and may be related to differential CYP2E1 induction. A comparison of the observed variation in V(maxC) values to other estimates of variability in the rate of oxidative metabolism and human CYP2E1 activity suggest a relatively narrow range in human hepatic activity toward DCM.
PMID:15501612 Sweeney LM et al; Toxicol Lett. 154(3):201-16 (2004).
Dichloromethane (DCM, methylene chloride) is a lipophilic volatile compound readily absorbed and then metabolized to several metabolites that may lead to chronic toxicity in different target organs. Physiologically based pharmacokinetic (PBPK) models are useful tools for calculation of internal and target organ doses of parent compound and metabolites. PBPK models, coupled with in vivo inhalation gas-uptake data, can be useful to estimate total metabolism. Previously, such an approach was used to make predictions regarding the metabolism and to make subsequent inferences of DCM's mode of action for toxicity. However, current evidence warrants re-examination of this approach. The goal of this work was to examine two different hypotheses for DCM metabolism in mice. One hypothesis describes two metabolic pathways: one involving cytochrome P450 2E1 (CYP2E1) and a second glutathione (GSH). The second metabolic hypothesis describes only one pathway mediated by CYP2E1 that includes multiple binding sites. The results of our analysis show that the in vivo gas-uptake data fit both hypotheses well and the traditional analysis of the chamber concentration data is not sufficient to distinguish between them. Gas-uptake data were re-analyzed by construction of a velocity plot as a function of increasing DCM initial concentration. The velocity (slope) analysis revealed that there are two substantially different phases in velocity, one rate for lower exposures and a different rate for higher exposures. The concept of a "metabolic switch," namely that due to conformational changes in the enzyme after one site is occupied - a different metabolic rate is seen - is also consistent with the experimental data. Our analyses raise questions concerning the importance of GSH metabolism for DCM. Recent research results also question the importance of this pathway in the toxicity of DCM. GSH-related DNA adducts were not formed after in vivo DCM exposure in mice and DCM-induced DNA damage has been detected in human lung cultures without GSH metabolism. In summary, a revised/updated metabolic hypothesis for DCM has been examined using in vivo inhalation data in mice combined with PBPK modeling that is consistent with up-to-date models of the active site for CYP2E1 and suggests that this pathway is the major metabolizing pathway for DCM metabolism.
PMID:20153349 Evans MV et al.; Toxicol Appl Pharmacol. 244(3):280-90 (2010).
Dichloromethane (DCM) is a hepatic and pulmonary carcinogen in mice exposed to high doses by inhalation. It has been shown previously that the incidence of liver and lung tumors does not increase in rats or hamsters exposed to the dihaloalkane under conditions similar to those that produced tumors in mice. The biological consequences of DCM exposure to humans is therefore uncertain. The carcinogenic effects of DCM in the mouse are caused by the interaction with DNA of a glutathione (GSH) conjugate that is produced by the class theta glutathione S-transferase T1-1 (GST T1-1). The species specificity is thought to be due to the greater amount of transferase activity in mouse target organs and specific nuclear localization of GST T1-1 in target cells. This paper directly compares the relative capacity and locality of DCM activation in mouse and human tissues. The results show that mouse GST T1-1 is more efficient in catalyzing the conjugation of DCM with GSH than the orthologous human enzyme. In addition, the mouse expresses higher levels of the transferase than humans in hepatic tissue. Histochemical analysis confirmed the presence of GST T1-1 in the nucleus of mouse liver cells. However, in human liver GST T1-1 was detected in bile duct epithelial cells and hepatocyte nuclei but was also present in the cytoplasm. Taking this information into account, it is unlikely that humans have a sufficiently high capacity to activate DCM for this compound to be considered to represent a carcinogenic risk.
PMID:11884241 Sherratt PJ et al; Toxicol Appl Pharmacol. 179(2):89-97 (2002).
... Biotransformation into carbon monoxide of dichloromethane ... by rat has been reported ... more recent studies of human exposure to dichloromethane in factory workers have confirmed these findings & have also demonstrated that incr expiration of carbon monoxide also occurs.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 4: A Review of the Literature Published during 1974 and 1975. London: The Chemical Society, 1977., p. 231
For more Metabolism/Metabolites (Complete) data for DICHLOROMETHANE (9 total), please visit the HSDB record page.
Methylene chloride has known human metabolites that include Dichloromethanol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
For carboxyhemoglobin in blood: 12-16 hours; [TDR, p. 862]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 862
Dichloromethane (DCM) elimination and carboxyhemoglobin (COHb) generation were examined in adult female SD rats pretreated with a glutathione (GSH) depletor(s). Rats were treated with either buthionine sulfoximine (BSO; 2 mmol/kg, i.p.), diethylmaleate (DEM; 3 mmol/kg, i.p.), phorone (PHO; 1 mmol/kg, i.p.) or BSO plus PHO (BSO; 2 mmol/kg +PHO; 0.5 mmol/kg, i.p.). ...The half-life of DCM in blood was also increased in rats pretreated with the GSH depletor(s).
PMID:11879980 Oh SJ et al; Toxicol Lett. 129(1-2):107-14 (2002).
When rats (male, Sprague-Dawley) were exposed to 50, 500, and 1500 ppm methylene chloride (dichloromethane, DCM) for 6 hr, plasma dichloromethane levels at apparent steady state were disproportionately higher with increasing exposure concn. Blood carboxyhemoglobin (HbCO) was 3% at 50 ppm and 10-13% at 500 ppm and at 1500 ppm. At the end of the 6 hr exposure, HbCO levels declined with half-life of 23 min.
PMID:6815830 McKenna MJ et al; Toxicol Appl Pharmacol 65 (1): 1-10 (1982)
The mechanism by which methylene chloride induces mammary adenomas in the rat is important for human hazard assessment. Female Sprague- Dawley rats receiving methylene chloride have a high blood level of prolactin. In common with the response to other agents which act via hyperprolactinaemia, the methylene chloride-induced response is of benign neoplasms only. There is no evidence for the binding of methylene chloride to the DNA of other tissues and hence it seems unlikely that it will bind to mammary tissue when the primary site of metabolism is the liver. It seems most likely, therefore, that the increased incidence of mammary adenomas is the result of an indirect mechanism operating via hyperprolactinaemia.
International Programme on Chemical Safety/ Environmental Health Criteria 164; Methylene Chloride (Second Edition). (1996). Available from, as of July 18, 2014: https://www.inchem.org/documents/ehc/ehc/ehc164.htm
Dichloromethane (DCM) is a hepatic and pulmonary carcinogen in mice exposed to high doses by inhalation. It has been shown previously that the incidence of liver and lung tumors does not increase in rats or hamsters exposed to the dihaloalkane under conditions similar to those that produced tumors in mice. The biological consequences of DCM exposure to humans is therefore uncertain. The carcinogenic effects of DCM in the mouse are caused by the interaction with DNA of a glutathione (GSH) conjugate that is produced by the class theta glutathione S-transferase T1-1 (GST T1-1). The species specificity is thought to be due to the greater amount of transferase activity in mouse target organs and specific nuclear localization of GST T1-1 in target cells. This paper directly compares the relative capacity and locality of DCM activation in mouse and human tissues. The results show that mouse GST T1-1 is more efficient in catalyzing the conjugation of DCM with GSH than the orthologous human enzyme. In addition, the mouse expresses higher levels of the transferase than humans in hepatic tissue. Histochemical analysis confirmed the presence of GST T1-1 in the nucleus of mouse liver cells. However, in human liver GST T1-1 was detected in bile duct epithelial cells and hepatocyte nuclei but was also present in the cytoplasm. Taking this information into account, it is unlikely that humans have a sufficiently high capacity to activate DCM for this compound to be considered to represent a carcinogenic risk.
PMID:11884241 Sherratt PJ et al; Toxicol Appl Pharmacol. 179(2):89-97 (2002).
Dichloromethane (DCM) is considered a probable human carcinogen. Laboratory studies have shown an increased incidence of lung and liver cancer in mice but not in rats or hamsters. Despite the correlation between metabolism of DCM by the glutathione-S-transferase (GST) pathway and the occurrence of tumors in different species, the mechanism of tumor induction by DCM metabolites produced through the GST pathway remains unclear. In this study a V79 cell line stably transfected with the murine GST theta 1 gene (mGSTT1) was compared to the parent cell line (MZ) to determine how the construct affects DCM metabolism and the sensitivity of the cell line to DNA damage and cytotoxicity. V79 cells were treated with DCM (2.5-10mM) or formaldehyde (150-600muM) for 2hr. Also, formaldehyde produced by V79 cytosol metabolism of DCM was measured spectrophotometrically. DNA damage and DNA-protein crosslinks were measured by the standard and proteinase K-modified alkaline single cell gel electrophoresis (SCG) assays. Cytotoxicity was assessed by trypan blue stain exclusion, the Live/Dead((R)) cell viability/cytotoxicity kit for animal cells, and the neutral red assay. After DCM treatment a significant concentration-dependent increase in tail moment in the V79 MZ cells was observed compared to a significant concentration-dependent decrease in tail moment in the V79 mGSTT1 cells. Post-incubation with proteinase K significantly increased DNA migrations in DCM-treated V79 mGSTT1 cells. DCM formed significantly higher levels of formaldehyde in the cytosol of the V79 mGSTT1 cells than in the cytosol of the V79 MZ cells. Results using the cytotoxicity assays were comparable using the trypan blue and Live/Dead((R)) assays, neither showing a difference in response between the two cell lines when exposed to either formaldehyde or DCM. These results indicate that V79 mGSTT1 can metabolize DCM to a genotoxic and cytotoxic metabolite, which is likely formaldehyde...
PMID:16765633 Hu Y et al; Mutat Res. 607(2):231-9 (2006).
The correlation between biol activity (toxicity and mutagenic effectiveness in Salmonella TA 100) and reactivity towards strong nucleophiles indicates that reactions with nucleophilic groups of high reactivity in biological materials, possibly SH or amino groups in proteins, are involved in dichloromethane's mechanism of action.
PMID:6352069 Osterman-Golkar S; Chem Biol Interact 46 (1): 121-30 (1983)
Increases in the concn of dichloromethane (DCM) lower the oxygen affinity of human hemoglobin as demonstrated by the shift of the oxygenation curves to higher partial pressures of oxygen and increase in the p50 (oxygen pressure necessary for fractional saturation of 0.50). Dichloromethane binds weakly to hemoglobin at four different sites, but binding to only one site is responsible for decreasing the oxygen affinity of hemoglobin.
PMID:7093282 Saxena AM et al; Biochem Biophys Acta 704: 1-6 (1982)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
95
PharmaCompass offers a list of Methylene Dichloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methylene Dichloride manufacturer or Methylene Dichloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methylene Dichloride manufacturer or Methylene Dichloride supplier.
PharmaCompass also assists you with knowing the Methylene Dichloride API Price utilized in the formulation of products. Methylene Dichloride API Price is not always fixed or binding as the Methylene Dichloride Price is obtained through a variety of data sources. The Methylene Dichloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Methylene Chloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Methylene Chloride, including repackagers and relabelers. The FDA regulates Methylene Chloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Methylene Chloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Methylene Chloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Methylene Chloride supplier is an individual or a company that provides Methylene Chloride active pharmaceutical ingredient (API) or Methylene Chloride finished formulations upon request. The Methylene Chloride suppliers may include Methylene Chloride API manufacturers, exporters, distributors and traders.
click here to find a list of Methylene Chloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Methylene Chloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Methylene Chloride active pharmaceutical ingredient (API) in detail. Different forms of Methylene Chloride DMFs exist exist since differing nations have different regulations, such as Methylene Chloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Methylene Chloride DMF submitted to regulatory agencies in the US is known as a USDMF. Methylene Chloride USDMF includes data on Methylene Chloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Methylene Chloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Methylene Chloride suppliers with USDMF on PharmaCompass.
Methylene Chloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Methylene Chloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Methylene Chloride GMP manufacturer or Methylene Chloride GMP API supplier for your needs.
A Methylene Chloride CoA (Certificate of Analysis) is a formal document that attests to Methylene Chloride's compliance with Methylene Chloride specifications and serves as a tool for batch-level quality control.
Methylene Chloride CoA mostly includes findings from lab analyses of a specific batch. For each Methylene Chloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Methylene Chloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Methylene Chloride EP), Methylene Chloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Methylene Chloride USP).
