
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
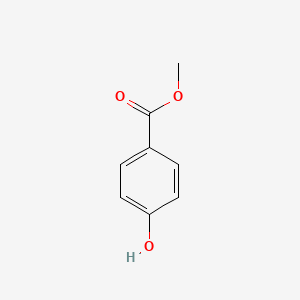

1. 4-hydroxybenzoic Acid Methyl Ester
2. Methyl P-hydroxybenzoate
3. Methyl-4-hydroxybenzoate
4. Methylparaben, Sodium Salt
5. Nipagin
1. Methyl 4-hydroxybenzoate
2. 99-76-3
3. Methyl Paraben
4. Methyl P-hydroxybenzoate
5. Nipagin
6. Methyl Parahydroxybenzoate
7. Tegosept M
8. Moldex
9. Maseptol
10. P-hydroxybenzoic Acid Methyl Ester
11. P-methoxycarbonylphenol
12. Benzoic Acid, 4-hydroxy-, Methyl Ester
13. P-carbomethoxyphenol
14. Preserval M
15. Methaben
16. Metoxyde
17. Preserval
18. Metaben
19. Paridol
20. Septos
21. Solbrol
22. Methyl Parasept
23. Methyl Butex
24. Methyl Chemosept
25. 4-hydroxybenzoic Acid Methyl Ester
26. Nipagin M
27. Aseptoform
28. Methylben
29. Abiol
30. Methyl-p-hydroxybenzoate
31. Methyl P-oxybenzoate
32. Solbrol M
33. 4-(methoxycarbonyl)phenol
34. 4-hydroxybenzoic Acid, Methyl Ester
35. Fema No. 2710
36. P-hydroxybenzoic Methyl Ester
37. Methyl Ester Of P-hydroxybenzoic Acid
38. P-oxybenzoesauremethylester
39. Mfcd00002352
40. Killitol
41. Benzoic Acid, P-hydroxy-, Methyl Ester
42. Methyl 4- Hydroxybenzoate
43. Nsc 3827
44. P-hydroxybenzoic Acid, Methyl Ester
45. Methylparaben E218
46. 4-hydroxy-benzoic Acid Methyl Ester
47. Nsc-3827
48. Methyl Paraben (e218)
49. Methylester Kyseliny P-hydroxybenzoove
50. Nsc-406127
51. A2i8c7hi9t
52. Chembl325372
53. Ins No.218
54. Chebi:31835
55. Ins-218
56. 4-hydroxybenzoic Acid-methyl Ester
57. Ncgc00159376-02
58. Ncgc00159376-04
59. E218
60. E-218
61. Dsstox_cid_2529
62. Wln: Qr Dvo1
63. Dsstox_rid_76616
64. Dsstox_gsid_22529
65. Methylparaben [usan]
66. Caswell No. 573pp
67. Fema Number 2710
68. 4-hydroxybenzoic Acid-methyl Ester 1000 Microg/ml In Acetonitrile
69. Cas-99-76-3
70. Smr000036660
71. Ccris 3946
72. Hsdb 1184
73. P-oxybenzoesauremethylester [german]
74. Einecs 202-785-7
75. Methylparaben [usan:nf]
76. Unii-a2i8c7hi9t
77. Epa Pesticide Chemical Code 061201
78. Brn 0509801
79. Metagin
80. Methyl4-hydroxybenzoate
81. Ai3-01336
82. Lexgard M
83. Paraben M
84. Methylester Kyseliny P-hydroxybenzoove [czech]
85. (methyl Paraben)
86. Methylparaben, Nf
87. Methylparaben, Fcc
88. 4-carbomethoxyphenol
89. Methylparaben (nf)
90. Methylparaben (tn)
91. Ins Number 218
92. Methylis Hydroxybenzoas
93. Solparol (salt/mix)
94. Methyl 4-hydoxybenzoate
95. Methyl 4 Hydroxybenzoate
96. Methyl 4-hydroxylbenzoate
97. Methyl 4-hydroxy-benzoate
98. Methyl-4-hydroxy-benzoate
99. Methyl 4-?hydroxybenzoate
100. Methylparaben [ii]
101. Methylparaben [mi]
102. Preserval Ms (salt/mix)
103. Bmse010009
104. Ec 202-785-7
105. Methyl (4-hydroxy)benzoate
106. Methylparaben [fcc]
107. Cid_7456
108. Schembl4440
109. Methylparaben [hsdb]
110. Methylparaben [inci]
111. Methylparaben [vandf]
112. Mls001304047
113. Mls001304187
114. Bidd:er0241
115. 4-hydroxybenzoate Methyl Ester
116. Methyl 4-hydroxybenzoate,(s)
117. Ins No. 218
118. Methyl Paraben [vandf]
119. Methylparaben [usp-rs]
120. Methylparaben [who-dd]
121. Amy901
122. Gtpl6273
123. Zinc1712
124. Methyl Parahydroxybenzoate (tn)
125. Dtxsid4022529
126. 4-hydroxybenzoic Acid Methylester
127. Methyl 4-hydroxybenzenecarboxylate
128. Nsc3827
129. Hms2883i08
130. Methyl Paraben, Analytical Standard
131. Methyl Parahydroxybenzoate (jp17)
132. 4-hydroxy Benzoic Acid Methyl Ester
133. Cs-d1181
134. Hy-n0349
135. Methyl Para Hydroxy Benzoate
136. Tox21_111616
137. Tox21_202318
138. Tox21_300009
139. Bbl005648
140. Bdbm50209100
141. Ck1194
142. Nsc406127
143. S3985
144. Stk802470
145. Akos000119910
146. Methyl Hydroxybenzoate [mart.]
147. Tox21_111616_1
148. Ccg-266228
149. Db14212
150. Methyl Hydroxybenzoate [who-ip]
151. Methyl P-hydroxybenzoate [fhfi]
152. Methyl Parahydroxybenzoate [jan]
153. Methyl 4-hydroxybenzoate, >=99%, Fcc
154. Ncgc00159376-03
155. Ncgc00159376-05
156. Ncgc00159376-06
157. Ncgc00253939-01
158. Ncgc00259867-01
159. Sy006626
160. Benzoic Acid,4-hydroxy,methyl Ester
161. Db-080628
162. Bb 0263150
163. E 218
164. Ft-0618697
165. Ft-0672044
166. H0216
167. M2206
168. Methyl 4-hydroxybenzoate, Analytical Standard
169. Methyl 4-hydroxybenzoate, P.a., 98-102%
170. Methylis Hydroxybenzoas [who-ip Latin]
171. D01400
172. Methyl 4-hydroxybenzoate, Usp, 98.0-102.0%
173. Methyl Parahydroxybenzoate [ep Monograph]
174. A846079
175. Methyl Salicylate Impurity C [ep Impurity]
176. Q229987
177. Q-200479
178. Methyl 4-hydroxybenzoate, Saj First Grade, >=98.0%
179. Methyl 4-hydroxybenzoate, Tested According To Ph.eur.
180. Propyl Hydroxybenzoate Impurity B [ep Impurity]
181. Z19674820
182. F1908-0119
183. Methyl 4-hydroxybenzoate, Bioxtra, >=99.0% (titration)
184. Methylparaben, Certified Reference Material, Tracecert(r)
185. 4-hydroxybenzoic Acid-methyl Ester 100 Microg/ml In Methanol
186. 4-hydroxybenzoic Acid-methyl Ester 1000 Microg/ml In Methanol
187. Methyl 4-hydroxybenzoate, Reagentplus(r), >=99.0%, Crystalline
188. Methylparaben, United States Pharmacopeia (usp) Reference Standard
189. Methyl 4-hydroxybenzoate, Bioreagent, Suitable For Insect Cell Culture
190. Methyl Parahydroxybenzoate, European Pharmacopoeia (ep) Reference Standard
191. Methylparaben, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 152.15 g/mol |
|---|---|
| Molecular Formula | C8H8O3 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 152.047344113 g/mol |
| Monoisotopic Mass | 152.047344113 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 136 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Preservatives, Pharmaceutical
Substances added to pharmaceutical preparations to protect them from chemical change or microbial action. They include ANTI-BACTERIAL AGENTS and antioxidants. (See all compounds classified as Preservatives, Pharmaceutical.)
By the oral route, parabens are rapidly absorbed, metabolized, and excreted. The metabolic reactions and conversions in mammals vary with the chain length of the ester, the animal species, route of administration, and quantity tested. The metabolism of parabens in humans appears to be most closely related to that of dogs. The rate of metabolite excretion appears to decrease with increasing molecular weight of the ester. /4-Hydroxybenzoates (Parabens)/
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V6 639
After methyl paraben is intravenously infused into the dog, nonhydrolyzed methyl paraben is found in brain, spleen, and pancreas. In liver, kidney, and muscle, it is immediately hydrolyzed to p-hydroxybenzoic acid ... Six hours after oral administration of 1.0 g/kg to dogs, the peak plasma concentration of free and total methyl paraben (630 and 867 ug/cu cm) is reached. After 48 hr, the vast majority was eliminated.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V6 664
The excretion and metabolism of methylparaben were monitored in 6 preterm infants after they had received multiple doses of a gentamicin formulation containing paraben preservatives. The recovery of the paraben from urine averaged 82.6%. The urinary excretion ranged from 13.2 to 88.1%.
PMID:6631690 Hindmarsh KW et al; J Pharm Sci 72: 1039-41 (1983)
... a study /was conducted/ using human volunteers in which the levels of methylparaben in the stratum corneum were measured. Cosmetic emulsions containing 0.15, 0.25, and 0.5% (w/v) methylparaben were applied one time to the forearm (42 sq cm) of one male and one female subject. At 1, 2, 5, and 12 hr after application, a small area was cleaned of emulsion using wet cotton and methylparaben was extracted by application of a glass cylinder (3.1 sq cm) with 0.5 mL ethanol for 25 min. Methylparaben concentrations were determined in the ethanol solvent using HPLC (for the 1, 2, and 5 hr durations) and GC/MS for other treatments. ... For the single application, methylparaben reached its peak 1 - 2 hr after application (peak was slightly higher for each higher use concentration) and returned to baseline after 12 hr. /In another study,/ healthy Japanese adults (one male, eleven female) applied a lotion only (6 subjects) or a lotion and an emulsion (6 subjects) containing Methylparaben (concentration not stated) twice a day for 1 month. Concentrations of methylparaben in the stratum corneum were determined as above using GC/MS before the first application, at 1, 2, 3, and 4 weeks, and 2 days after stopping. ... Repeated applications resulted in an increase in methylparaben concentration in the stratum corneum over time for both the lotion application and the lotion plus emulsion application. After 2 days, methylparaben had returned to pretreatment levels.
Cosmetic Ingredient Review; Final Amended Report on the Safety Assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in Cosmetic Products p 25. Int J Toxicol 27 Suppl 4: 1-82 (2008). Available from, as of November 21, 2016: https://online.personalcarecouncil.org/ctfa-static/online/lists/cir-pdfs/PR427.pdf
For more Absorption, Distribution and Excretion (Complete) data for METHYLPARABEN (7 total), please visit the HSDB record page.
In mice, rats, rabbits, or dogs methylparaben is excreted in the urine as unchanged benzoate, p-hydroxybenzoic acid, p-hydroxyhippuric acid (p-hydroxybenzoylglycine), ester glucuronides, ether glucuronides, or ether sulfates.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V6 665
By the oral route, parabens are rapidly absorbed, metabolized, and excreted. The metabolic reactions and conversions in mammals vary with the chain length of the ester, the animal species, route of administration, and quantity tested. The metabolism of parabens in humans appears to be most closely related to that of dogs. The rate of metabolite excretion appears to decrease with increasing molecular weight of the ester. /4-Hydroxybenzoates (Parabens)/
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V6 639
/This study examined/ the metabolic fate of methylparaben in rabbits. The compound was given by gastric intubation, and urine was analyzed by paper chromatography. Three major metabolites, p-hydroxybenzoic acid, p-hydroxyhippuric acid, and p-carboxyphenyl glucuronide, as well as two minor metabolites, p-hydroxybenzoyl glucuronide and p-carboxyphenyl sulfate, were identified. Rabbits given orally 0.4 or 0.8 g/kg methylparaben, ethylparaben, propylparaben, or butylparaben excreted only 0.2 to 0.9% of the unchanged ester by 24 hr. Urinary excretion of p-hydroxybenzoic acid was slower with increasing carbon chain length of the paraben alkyl group. Excretion of the conjugated acid was approximately that of the free acid. At 24 hr following paraben administration, 25 to 39% was recovered as p-hydroxybenzoic acid, 15 to 29% as the glycine conjugate, 5 to 8% as the ester glucuronide, 10 to 18% as the ether glucuronide, and 7 to 12% as the sulfate.
Cosmetic Ingredient Review; Final Amended Report on the Safety Assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in Cosmetic Products p 27. Int J Toxicol 27 Suppl 4: 1-82 (2008). Available from, as of November 21, 2016: https://online.personalcarecouncil.org/ctfa-static/online/lists/cir-pdfs/PR427.pdf
The metabolism of methylparaben, ethylparaben, and propylparaben was studied in rats. Animals were given orally 100 mg of ester. Blood and urine were collected regularly and analyzed by paper chromatography. Paraben metabolites were identified in the urine 30 minutes after dosing. No unchanged paraben was detected. Ninety minutes after dosing, excretion of metabolites was maximum; thereafter, excretion decreased. p-Hydroxyhippuric acid appeared in the urine after 30 minutes; its concentration then increased evenly during the next 4 hr. The glucuronide and ethereal sulfate metabolites appeared only between 30 and 75 minutes postingestion. After 90 minutes, 67 to 75% of the total paraben dose was excreted as p-hydroxybenzoic acid, 10 to 12.5% as p-hydroxyhippuric acid, and 8 to 10% as glucuronyl derivatives. The concentration of free p-hydroxybenzoic acid in the blood remained extremely low. A continuous rise occurred within the first hour, but the concentration thereafter decreased and leveled off 1 to 2 hr after ingestion. The authors concluded that there were two stages of paraben detoxification: (1) absorption of paraben and excretion in urine of p-hydroxybenzoic acid, and (2) metabolic detoxification by glucuronic-, sulfo-, and glycino-conjugation.
Cosmetic Ingredient Review; Final Amended Report on the Safety Assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in Cosmetic Products p 27. Int J Toxicol 27 Suppl 4: 1-82 (2008). Available from, as of November 21, 2016: https://online.personalcarecouncil.org/ctfa-static/online/lists/cir-pdfs/PR427.pdf
For more Metabolism/Metabolites (Complete) data for METHYLPARABEN (10 total), please visit the HSDB record page.
...The mechanism of cytotoxic action of parabens may be linked to mitochondrial failure dependent on induction of membrane permeability transition accompanied by the mitochondrial depolarization and depletion of cellular ATP through uncoupling of oxidative phosphorylation.
PMID:12387298 Soni MG et al; Food Chem Toxicol 40 (10): 1335-73 (2002)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
100
PharmaCompass offers a list of Methyl 4-Hydroxybenzoate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methyl 4-Hydroxybenzoate manufacturer or Methyl 4-Hydroxybenzoate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methyl 4-Hydroxybenzoate manufacturer or Methyl 4-Hydroxybenzoate supplier.
PharmaCompass also assists you with knowing the Methyl 4-Hydroxybenzoate API Price utilized in the formulation of products. Methyl 4-Hydroxybenzoate API Price is not always fixed or binding as the Methyl 4-Hydroxybenzoate Price is obtained through a variety of data sources. The Methyl 4-Hydroxybenzoate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A methylparaben manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of methylparaben, including repackagers and relabelers. The FDA regulates methylparaben manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. methylparaben API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of methylparaben manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A methylparaben supplier is an individual or a company that provides methylparaben active pharmaceutical ingredient (API) or methylparaben finished formulations upon request. The methylparaben suppliers may include methylparaben API manufacturers, exporters, distributors and traders.
click here to find a list of methylparaben suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing methylparaben as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for methylparaben API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture methylparaben as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain methylparaben and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a methylparaben NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of methylparaben suppliers with NDC on PharmaCompass.
methylparaben Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of methylparaben GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right methylparaben GMP manufacturer or methylparaben GMP API supplier for your needs.
A methylparaben CoA (Certificate of Analysis) is a formal document that attests to methylparaben's compliance with methylparaben specifications and serves as a tool for batch-level quality control.
methylparaben CoA mostly includes findings from lab analyses of a specific batch. For each methylparaben CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
methylparaben may be tested according to a variety of international standards, such as European Pharmacopoeia (methylparaben EP), methylparaben JP (Japanese Pharmacopeia) and the US Pharmacopoeia (methylparaben USP).
