
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
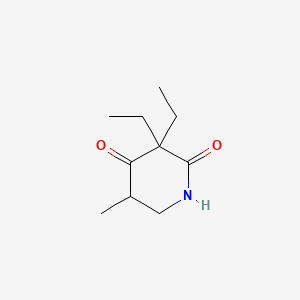

1. Methprylon
2. Noludar
1. Metiprilone
2. Noludar
3. Metiprilon
4. Dimerin
5. Noctan
6. Methyprylone
7. Methyprolon
8. Methyprylon [inn]
9. 125-64-4
10. Methyprylonum
11. Methprylon
12. Nodular
13. Methyprylone [inn-french]
14. Methyprylonum [inn-latin]
15. Metiprilona [inn-spanish]
16. 3,3-diethyl-5-methylpiperidine-2,4-dione
17. Ro 1-6463
18. 2,4-piperidinedione, 3,3-diethyl-5-methyl-
19. 2,4-dioxo-3,3-diethyl-5-methylpiperidine
20. 3,3-diethyl-5-methyl-2,4-piperidinedione
21. Dea No. 2575
22. 2,4-dioxy-3,3-diethyl-5-methylpiperidine
23. 3,3-diethyl-2,4-dioxo-5-methylpiperidine
24. Nsc 30442
25. Methyprylon, (+)-
26. Methyprylon, (-)-
27. Nsc-30442
28. Cut48i42on
29. P66z3ur32t
30. X66m7yn35v
31. Metiprilona
32. 2,4-piperidinedione, 3,3-diethyl-5-methyl-, (+)-
33. 2,4-piperidinedione, 3,3-diethyl-5-methyl-, (-)-
34. 30590-00-2
35. 30590-01-3
36. Noludar (tn)
37. Hsdb 3128
38. Methyprylon (jan/inn)
39. Einecs 204-745-4
40. Unii-cut48i42on
41. Brn 0082860
42. Methyprylon [usp:inn:ban]
43. (+)-noludar
44. (?)-noludar
45. Methyprylon [mi]
46. Methyprylon [jan]
47. Methyprylon [hsdb]
48. Methyprylon [vandf]
49. Methyprylon [mart.]
50. Unii-p66z3ur32t
51. Unii-x66m7yn35v
52. Methyprylon [who-dd]
53. Schembl155838
54. 3,4-dioxo-5-methylpiperidine
55. 2, 3,3-diethyl-5-methyl-
56. Gtpl7238
57. 2,3-diethyl-5-methylpiperidine
58. Chembl1200790
59. Dtxsid7023306
60. Chebi:31837
61. Methyprylon [orange Book]
62. Nsc30442
63. Wln: T6mv Dvtj C2 C2 E1
64. Akos006239549
65. Db01107
66. D01150
67. Q409558
68. 5,5-diethyl-6-hydroxy-3-methyl-2,3,4,5-tetrahydropyridin-4-one
| Molecular Weight | 183.25 g/mol |
|---|---|
| Molecular Formula | C10H17NO2 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 183.125928785 g/mol |
| Monoisotopic Mass | 183.125928785 g/mol |
| Topological Polar Surface Area | 46.2 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 231 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
HYPNOTIC USEFUL IN MGMNT OF INSOMNIA OF VARIED ETIOLOGY. IT USUALLY INDUCES SLEEP WITHIN 45 MIN; IT PROVIDES SLEEP FOR 5-8 HR. HENCE, IT IS USEFUL AS HYPNOTIC IN PT WITH SIMPLE & NERVOUS INSOMNIA.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1007
300 MG DOSE ... SUPPRESSES REM SLEEP AS MUCH AS DOES 100 MG OF PENTOBARBITAL.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 134
The Primary Medication Classification of the US Veterans Administration is CN309: Sedatives/Hypnotics, Other
United States Pharmacopeial Convention; USP Dispensing Information 12th ed Vol IB p.1900 (1992)
For more Therapeutic Uses (Complete) data for METHYPRYLON (6 total), please visit the HSDB record page.
May be habit forming.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 964
ABSTINENCE SYNDROME IS LIKE THAT OF BARBITURATES & INCL INSOMNIA, CONFUSION, HALLUCINATIONS, & CONVULSIONS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 134
PATIENTS SHOULD BE WARNED AGAINST CONCOMITANT USE OF METHYPRYLON & ALCOHOL OR OTHER CNS DEPRESSANTS. ... ITS SAFE USE DURING PREGNANCY & IN CHILDREN UNDER 3 YR OF AGE HAS NOT BEEN ESTABLISHED.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1007
SIDE EFFECTS ARE USUALLY INFREQUENT & MILD. THERE HAVE BEEN RARE CASES OF MORNING DROWSINESS, DIZZINESS ... ESOPHAGITIS ...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1007
For more Drug Warnings (Complete) data for METHYPRYLON (9 total), please visit the HSDB record page.
... IN FATAL CASES, BLOOD CONCN IN EXCESS OF 9.0 MG/100 ML HAVE BEEN REPORTED.
Sunshine, Irving (ed.) Methodology for Analytical Toxicology. Cleveland: CRC Press, Inc., 1975., p. 261
For the treatment of insomnia.
Methyprylon, a piperidinedione CNS depressant, is close to barbituric acid in structure, but different enough to be called a "non-barbiturate" sedative-hynotic. Methyprylon is used for insomnia and daytime tension. Methyprylon depresses the activity of muscle tissues, the heart, and the respiratory system.
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CE - Piperidinedione derivatives
N05CE02 - Methyprylon
DRUG IS WELL ABSORBED FROM INTESTINE.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 69
IT IS MORE WATER SOL THAN GLUTETHIMIDE, BUT IT IS NOT KNOWN WHETHER GI ABSORPTION IS CONSEQUENTLY LESS ERRATIC. ... ONLY 3% IS EXCRETED IN URINE UNCHANGED. ... ONLY 60% OF FREE METABOLITES & GLUCURONIDES IS RECOVERABLE FROM URINE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 134
THERAPEUTIC BLOOD CONCN PROBABLY DO NOT EXCEED 1.0 MG/100 ML. FOLLOWING SINGLE ORAL DOSE OF 650 MG IN ADULTS, MAX PLASMA CONCN OF APPROX 1.0 MG/100 ML IS REACHED AFTER 2 HR. IN FATAL CASES, BLOOD CONCN IN EXCESS OF 9.0 MG/100 ML HAVE BEEN REPORTED.
Sunshine, Irving (ed.) Methodology for Analytical Toxicology. Cleveland: CRC Press, Inc., 1975., p. 261
Methyprylon does not accumulate selectively in organ systems.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 852
For more Absorption, Distribution and Excretion (Complete) data for METHYPRYLON (6 total), please visit the HSDB record page.
Hepatic. Methyprylon is almost completely metabolized.
FROM URINE OF HUMAN SUBJECTS ... TAKEN METHYPRYLON ... NEW METABOLITE HAS BEEN ... IDENTIFIED AS 2,4,6-TRIOXO-3,3-DIETHYL-5-METHYLPIPERIDINE. 2,4-DIOXO-3,3-DIETHYL-5-METHYLTETRAHYDROPYRIDINE, 2,4-DIOXO-3,3-DIETHYL-5-HYDROXYMETHYLTETRAHYDROPYRIDINE, & 4,6-DIOXO-5,5-DIETHYL-TETRAHYDRONICOTINIC ACID ... ESTABLISHED AS METABOLITES ...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 155
While the metabolic degradation of methyprylon is known, it is not certain in which organ this takes place. Methyprylon is dehydrogenated to 5-methyl-pyrithyldione, then partially oxidized to hydroxy derivatives, and finally oxidized to 5-carboxy-pyrithyldione. The hydroxy derivatives are bound approximately 38% to human plasma proteins. Other less important metabolites may arise, but the metabolites are partly conjugated to glucuronide, with approximately 97% of the parent drug being metabolized. Intact methyprylon (3%) and its metabolites (60%) are recovered in the urine, while approximately 20% is found in the feces, demonstrating that enterohepatic circulation of the drug occurs.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 851
6-16 hours
PLASMA T1/2 IS 4 HR, BUT IT IS LONGER IN ACUTE INTOXICATION.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 366
Plasma half-life is 6 to 16 hours. Onset of action within 45 minutes. Duration of action 5 to 8 hours.
United States Pharmacopeial Convention; USP Dispensing Information 12th ed Vol IB p.1900 (1992)
Biologic half-life is not well defined, but varies between 4 and 16 hours, although a longer action has been observed in severely poisoned patients.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 851
Methyprylon binds at a distinct binding site associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged.
INCREASED STEROID HYDROXYLATION IS PROBABLY ONE IMPORTANT MECHANISM OF ACTION OF SO-CALLED CATATOXIC STEROIDS. SUCH CMPD HAVE BEEN REPORTED TO PROTECT ANIMALS AGAINST ACUTE TOXIC EFFECTS OF EXTRAORDINARY VARIETY OF DRUGS ... METHYPRYLON.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 608
METHYPRYLON STIMULATES HEPATIC MICROSOMAL ENZYME SYSTEM & DELTA-ALA SYNTHETASE ...
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 366
ABOUT THIS PAGE
22
PharmaCompass offers a list of Methyprylon API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Methyprylon manufacturer or Methyprylon supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Methyprylon manufacturer or Methyprylon supplier.
PharmaCompass also assists you with knowing the Methyprylon API Price utilized in the formulation of products. Methyprylon API Price is not always fixed or binding as the Methyprylon Price is obtained through a variety of data sources. The Methyprylon Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Methyprylon manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Methyprylon, including repackagers and relabelers. The FDA regulates Methyprylon manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Methyprylon API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Methyprylon supplier is an individual or a company that provides Methyprylon active pharmaceutical ingredient (API) or Methyprylon finished formulations upon request. The Methyprylon suppliers may include Methyprylon API manufacturers, exporters, distributors and traders.
Methyprylon Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Methyprylon GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Methyprylon GMP manufacturer or Methyprylon GMP API supplier for your needs.
A Methyprylon CoA (Certificate of Analysis) is a formal document that attests to Methyprylon's compliance with Methyprylon specifications and serves as a tool for batch-level quality control.
Methyprylon CoA mostly includes findings from lab analyses of a specific batch. For each Methyprylon CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Methyprylon may be tested according to a variety of international standards, such as European Pharmacopoeia (Methyprylon EP), Methyprylon JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Methyprylon USP).
