
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
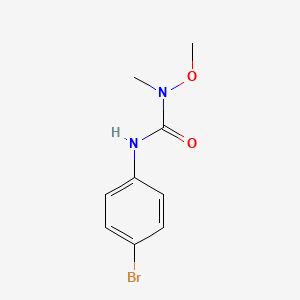

1. 3-(p-bromophenyl)-1-methoxy-1-methylurea
2. Patoran
1. 3060-89-7
2. Metbromuron
3. Patoran
4. 3-(4-bromophenyl)-1-methoxy-1-methylurea
5. Metobromurone
6. Monobromuron
7. Pattonex
8. Urea, N'-(4-bromophenyl)-n-methoxy-n-methyl-
9. N'-(4-bromophenyl)-n-methoxy-n-methylurea
10. Ciba-3126
11. 3-(p-bromophenyl)-1-methoxy-1-methylurea
12. Urea, 3-(p-bromophenyl)-1-methoxy-1-methyl-
13. 3-(p-bromophenyl)-1-methyl-1-methoxyurea
14. 3-(4-bromphenyl)-1-methoxyharnstoff
15. Chebi:81964
16. Metobromuron 10 Microg/ml In Acetonitrile
17. Metobromuron 100 Microg/ml In Acetonitrile
18. 4251089p3l
19. Caswell No. 579a
20. Metobromuron [iso]
21. Ccris 6765
22. Metobromuron [ansi:bsi:iso]
23. Hsdb 1741
24. Einecs 221-301-5
25. Epa Pesticide Chemical Code 035901
26. Brn 2103964
27. Patoran Fl
28. 3-(4-bromphenyl)-1-methoxyharnstoff [german]
29. C-3126
30. Unii-4251089p3l
31. N-(4-bromophenyl)-n'-methyl-n'-methoxy-harnstoff [german]
32. 1-(4-bromophenyl)-3-methoxy-3-methylurea
33. Metobromuron [mi]
34. Metobromuron [hsdb]
35. Dsstox_cid_22157
36. Dsstox_rid_79940
37. Dsstox_gsid_42157
38. Schembl53871
39. N-(4-bromophenyl)-n'-methyl-n'-methoxy-harnstoff
40. Chembl1356637
41. Dtxsid6042157
42. Metobromuron, Analytical Standard
43. Zinc260866
44. Dndi1246791
45. Tox21_301964
46. Akos001609255
47. Ncgc00166156-01
48. Ncgc00255893-01
49. Cas-3060-89-7
50. Db-047812
51. Ft-0603626
52. N-(p-bromophenyl)-n'-methyl-n'-methoxyurea
53. C18793
54. Metobromuron, Pestanal(r), Analytical Standard
55. 060m897
56. J-018035
57. Q18213352
58. Pesticide3_metobromuron_c9h11brn2o2_urea, N'-(4-bromophenyl)-n-methoxy-n-methyl-
| Molecular Weight | 259.10 g/mol |
|---|---|
| Molecular Formula | C9H11BrN2O2 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 258.00039 g/mol |
| Monoisotopic Mass | 258.00039 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 193 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
METOBROMURON IS ABSORBED BY ROOTS AND LEAVES ...
Worthing, C. R. (ed.). Pesticide Manual. 6th ed. Worcestershire, England: British Crop Protection Council, l979., p. 359
... Different urea herbicides exhibit different mobilities in plant systems. ... Uptake and translocation of chloroxuron, fluometuron, and metobromuron by bean plants when supplied to nutrient cultures in equal concn (essentially equimolar) and specific radioactivities were studied. Whereas the movement of chloroxuron to aerial parts was found to be restricted under the short-term conditions applied, both fluometuron and metobromuron were rapidly translocated into leaves. However, the two latter cmpd differed strikingly with regard to their distribution pattern within the leaves. In contrast to metobromuron, which was mainly confined to tracheal veins, fluometuron had almost completely moved out into the mesophyll tissues. Although no extensive comparisons of the movement of different urea structures are available at this time, it would appear that their apoplastic distribution in plants is regulated in part by the same physical-chemical phenomena which control the mobility and availability of the cmpd in soil.
Spencer, E. Y. Guide to the Chemicals Used in Crop Protection. 7th ed. Publication 1093. Research Institute, Agriculture Canada, Ottawa, Canada: Information Canada, 1982., p. 235
METOBROMURON IS METABOLIZED IN PLANTS & ANIMALS BY N-DEMETHYLATION & N-DEMETHOXYLATION, RESPECTIVELY.
Martin, H. and C.R. Worthing (eds.). Pesticide Manual. 4th ed. Worcestershire, England: British Crop Protection Council, 1974., p. 355
... RING-LABELED (14)C-METOBROMURON WAS SUPPLIED TO POTATOES AND CORN SEEDLINGS. ... FOLLOWING METABOLITES ... /WERE IDENTIFIED/: 3-(4-BROMOPHENYL)-1-METHOXYUREA, 3-(4-BROMOPHENYL)-1-METHYLUREA, AND 4-BROMOPHENYLUREA.
Kearney, P.C., and D.D. Kaufman. Degradation of Herbicides. New York: Marcel Dekker, Inc., 1969., p. 99
METOBROMURON YIELDS 3-(P-BROMOPHENYL)-1-METHYLUREA AND 3-(P-BROMOPHENYL)-1-METHOXYUREA IN TALAROMYCES. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. M45
(14)C-Metobromuron was incubated with human embryonic lung (HEL) cells. More than 95% of the label was recovered as unchanged metobromuron. Of four metabolites, three were identified: 3-(4-bromophenyl)-1-methylurea; 4-bromophenylurea; and 3-(4-bromophenyl)-1-methylurea.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 563
ABOUT THIS PAGE
48
PharmaCompass offers a list of Metobromuron API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Metobromuron manufacturer or Metobromuron supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Metobromuron manufacturer or Metobromuron supplier.
PharmaCompass also assists you with knowing the Metobromuron API Price utilized in the formulation of products. Metobromuron API Price is not always fixed or binding as the Metobromuron Price is obtained through a variety of data sources. The Metobromuron Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Metobromuron manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Metobromuron, including repackagers and relabelers. The FDA regulates Metobromuron manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Metobromuron API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Metobromuron supplier is an individual or a company that provides Metobromuron active pharmaceutical ingredient (API) or Metobromuron finished formulations upon request. The Metobromuron suppliers may include Metobromuron API manufacturers, exporters, distributors and traders.
Metobromuron Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Metobromuron GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Metobromuron GMP manufacturer or Metobromuron GMP API supplier for your needs.
A Metobromuron CoA (Certificate of Analysis) is a formal document that attests to Metobromuron's compliance with Metobromuron specifications and serves as a tool for batch-level quality control.
Metobromuron CoA mostly includes findings from lab analyses of a specific batch. For each Metobromuron CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Metobromuron may be tested according to a variety of international standards, such as European Pharmacopoeia (Metobromuron EP), Metobromuron JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Metobromuron USP).
