
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
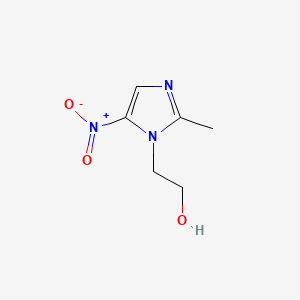

1. 2 Methyl 5 Nitroimidazole 1 Ethanol
2. 2-methyl-5-nitroimidazole-1-ethanol
3. Bayer 5360
4. Clont
5. Danizol
6. Flagyl
7. Gineflavir
8. Metric
9. Metrodzhil
10. Metrogel
11. Metrogyl
12. Metronidazole Hydrochloride
13. Metronidazole Monohydrochloride
14. Metronidazole Phosphate
15. Metronidazole Phosphoester
16. Satric
17. Trichazol
18. Trichopol
19. Trivazol
20. Vagilen
1. 443-48-1
2. Flagyl
3. Metronidazol
4. 2-methyl-5-nitroimidazole-1-ethanol
5. Anagiardil
6. Gineflavir
7. Metrogel
8. Deflamon
9. Orvagil
10. Trichazol
11. Bayer 5360
12. Meronidal
13. Metronidaz
14. Novonidazol
15. Trichopol
16. Trivazol
17. Danizol
18. Mexibol
19. Vagilen
20. Clont
21. Flagemona
22. Giatricol
23. Metronidazolo
24. Protostat
25. Sanatrichom
26. Takimetol
27. Trichocide
28. Trichomol
29. Trikacide
30. Acromona
31. Atrivyl
32. Efloran
33. Entizol
34. Flagesol
35. Monagyl
36. Monasin
37. Trichex
38. Tricocet
39. Trikamon
40. Trikojol
41. Trikozol
42. Trimeks
43. Vagimid
44. Vertisal
45. Wagitran
46. Arilin
47. Bexon
48. Elyzol
49. Eumin
50. Flagil
51. Klion
52. Klont
53. Metrocream
54. Metrolotion
55. Nalox
56. Satric
57. Tricom
58. 2-(2-methyl-5-nitro-1h-imidazol-1-yl)ethanol
59. Neo-tric
60. Tricowas B
61. Metrogel-vaginal
62. Deflamon-wirkstoff
63. Metromidol
64. Cont
65. Nida
66. Methronidazole
67. Noritate
68. Trichopal
69. Flegyl
70. Fossyol
71. Flagyl Er
72. Metro I.v.
73. Metronidazolum
74. 1h-imidazole-1-ethanol, 2-methyl-5-nitro-
75. Metrolyl
76. Vandazole
77. Zadstat
78. Metric 21
79. Rp 8823
80. Trichomonacid 'pharmachim'
81. 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole
82. Nsc-50364
83. 2-(2-methyl-5-nitroimidazol-1-yl)ethanol
84. Metronidazole In Plastic Container
85. 2-methyl-1-(2-hydroxyethyl)-5-nitroimidazole
86. 2-methyl-3-(2-hydroxyethyl)-4-nitroimidazole
87. Sc 10295
88. 1-(beta-ethylol)-2-methyl-5-nitro-3-azapyrrole
89. 1-(2-hydroxy-1-ethyl)-2-methyl-5-nitroimidazole
90. 2-(2-methyl-5-nitro-1h-imidazol-1-yl)ethan-1-ol
91. 2-(2-methyl-5-nitro-1-imidazolyl)ethanol
92. Mfcd00009750
93. 1-(beta-hydroxyethyl)-2-methyl-5-nitroimidazole
94. 1-hydroxyethyl-2-methyl-5-nitroimidazole
95. Flagyl I.v. Rtu In Plastic Container
96. 1-(beta-oxyethyl)-2-methyl-5-nitroimidazole
97. Nsc 50364
98. Imidazole-1-ethanol, 2-methyl-5-nitro-
99. 2-(2-methyl-5-nitro-imidazol-1-yl)ethanol
100. Bay-5360
101. Nsc69587
102. Nsc-69587
103. Rp-8823
104. Mls000028590
105. Bayer-5360
106. Chebi:6909
107. 140qmo216e
108. Metro Gel
109. Nsc50364
110. Ncgc00016446-06
111. Cas-443-48-1
112. Metrolag
113. Metrotop
114. Rathimed
115. Smr000058175
116. Tricho Cordes
117. Dsstox_cid_892
118. Metronidazolo [dcit]
119. Tricho-gynaedron
120. Dsstox_rid_75848
121. Dsstox_gsid_20892
122. Mexibol 'silanes'
123. Metro I.v. In Plastic Container
124. 1-(.beta.-ethylol)-2-methyl-5-nitro-3-azapyrrole
125. 1-(.beta.-hydroxyethyl)-2-methyl-5-nitroimidazole
126. Metronidazol [inn-spanish]
127. Metronidazolum [inn-latin]
128. Flagyl I.v. Rtu
129. Flagyl 375
130. Trichobrol
131. Florazole
132. Mepagyl
133. Nidagyl
134. Rosased
135. Zidoval
136. Caswell No. 579aa
137. Wln: T5n Cnj A2q B1 Enw
138. Noritate (tn)
139. Ccris 410
140. Metro Cream & Gel
141. Flagyl (tn)
142. Hsdb 3129
143. Wln: T6ntj Dq Anu1- Et5n Cnj A1 Bnw
144. Sr-01000000244
145. Einecs 207-136-1
146. Nsc 69587
147. Epa Pesticide Chemical Code 120401
148. Brn 0611683
149. Polibiotic
150. Trikhopol
151. Donnan
152. Flazol
153. Unii-140qmo216e
154. Cb-01-14 Mmx
155. Metro Iv
156. Vandazole (tn)
157. Metronidazole,(s)
158. Prestwick_334
159. Nuvessa (tn)
160. Idr-90105
161. Cimetrol 500lpci
162. Metronidazole Solution
163. Metronidazole, Bioxtra
164. Metronidazole (flagyl)
165. Spectrum_001035
166. Metronidazole [usan:usp:inn:ban:jan]
167. Helidac (salt/mix)
168. 2-(2-methyl-5-nitroimidazolyl)ethan-1-ol
169. Maybridge1_001999
170. Opera_id_1585
171. Prestwick0_000081
172. Prestwick1_000081
173. Prestwick2_000081
174. Prestwick3_000081
175. Spectrum2_000883
176. Spectrum3_000506
177. Spectrum4_000060
178. Spectrum5_001289
179. M0924
180. Metronidazole [mi]
181. Chembl137
182. Metronidazole [inn]
183. Metronidazole [jan]
184. Metronidazole [hsdb]
185. Metronidazole [iarc]
186. Metronidazole [usan]
187. Nciopen2_000337
188. Schembl23042
189. Bspbio_000002
190. Bspbio_002031
191. Kbiogr_000559
192. Kbioss_001515
193. Metronidazole [vandf]
194. 5-23-05-00063 (beilstein Handbook Reference)
195. Mls000758286
196. Mls001424018
197. Bidd:gt0107
198. Divk1c_000007
199. Metronidazole [mart.]
200. Spectrum1500412
201. Spbio_000666
202. Spbio_001941
203. Metronidazole [usp-rs]
204. Metronidazole [who-dd]
205. Metronidazole [who-ip]
206. Bpbio1_000004
207. Dtxsid2020892
208. Flagyl I.v. Rtu (salt/mix)
209. Bcbcmap01_000184
210. Gtpl10914
211. Hms500a09
212. Hms547c19
213. Kbio1_000007
214. Kbio2_001515
215. Kbio2_004083
216. Kbio2_006651
217. Kbio3_001531
218. Metronidazole (jp17/usp/inn)
219. Metronidazole, Analytical Standard
220. Ninds_000007
221. Hms1568a04
222. Hms1920n19
223. Hms2051g07
224. Hms2090b19
225. Hms2091f14
226. Hms2095a04
227. Hms2231e11
228. Hms3373o05
229. Hms3393g07
230. Hms3655e22
231. Hms3712a04
232. Pharmakon1600-01500412
233. Zinc113442
234. Metronidazole [ep Impurity]
235. Metronidazole [orange Book]
236. Bcp13757
237. Hy-b0318
238. Metronidazole [ep Monograph]
239. Metronidazole [usp Impurity]
240. Tox21_110441
241. Tox21_202413
242. Tox21_302794
243. Bbl005452
244. Bdbm50375309
245. Ccg-40016
246. Fp-250
247. Metronidazole [usp Monograph]
248. Nsc757118
249. Pylera Component Metronidazole
250. S1907
251. Stk177359
252. Helidac Component Metronidazole
253. Metronidazole 2.0 Mg/ml In Methanol
254. Akos000269646
255. Akos005169650
256. Tox21_110441_1
257. Db00916
258. Ks-5140
259. Nc00020
260. Nsc-757118
261. Idi1_000007
262. Metronidazole Component Of Pylera
263. Smp1_000189
264. Metronidazole Component Of Helidac
265. Ncgc00016446-01
266. Ncgc00016446-02
267. Ncgc00016446-03
268. Ncgc00016446-04
269. Ncgc00016446-05
270. Ncgc00016446-07
271. Ncgc00016446-08
272. Ncgc00016446-09
273. Ncgc00016446-11
274. Ncgc00016446-12
275. Ncgc00016446-17
276. Ncgc00022059-03
277. Ncgc00022059-04
278. Ncgc00022059-05
279. Ncgc00256513-01
280. Ncgc00259962-01
281. Ac-23968
282. Sy002821
283. Metronidazole 1000 Microg/ml In Methanol
284. Sbi-0051447.p003
285. Db-051212
286. Metronidazole, Saj First Grade, >=99.0%
287. Ab00052046
288. Bb 0218386
289. Ft-0603394
290. Metronidazole 100 Microg/ml In Acetonitrile
291. Sw196613-4
292. C07203
293. D00409
294. Ab00052046-17
295. Ab00052046_18
296. Ab00052046_19
297. A826552
298. Metronidazole, Vetranal(tm), Analytical Standard
299. Q169569
300. 2-(2-methyl-5-nitro-1h-imidazol-1-yl)-1-ethanol
301. 2-(2-methyl-5-nitro-1h-imidazol-1-yl)ethanol #
302. Metronidazole, Antibiotic For Culture Media Use Only
303. Q-201403
304. Sr-01000000244-4
305. Sr-01000000244-5
306. Brd-k52020312-001-05-2
307. Brd-k52020312-001-15-1
308. Metronidazole Benzoate Impurity A [ep Impurity]
309. Z87001124
310. F1773-0073
311. Metronidazole, Certified Reference Material, Tracecert(r)
312. Metronidazole, British Pharmacopoeia (bp) Reference Standard
313. Metronidazole, European Pharmacopoeia (ep) Reference Standard
314. Metronidazole, United States Pharmacopeia (usp) Reference Standard
315. Metronidazole Solution, 2.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
316. Metronidazole, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 171.15 g/mol |
|---|---|
| Molecular Formula | C6H9N3O3 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 171.06439116 g/mol |
| Monoisotopic Mass | 171.06439116 g/mol |
| Topological Polar Surface Area | 83.9 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 170 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 24 | |
|---|---|
| Drug Name | Flagyl |
| PubMed Health | Metronidazole |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antiacne Antibacterial, Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori |
| Drug Label | FLAGYL (metronidazole) tablets, 250 mg or 500 mg is an oral formulation of the synthetic nitroimidazole antimicrobial, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural formula:FLAGYL (metronidazole) tablets contain 250 mg o... |
| Active Ingredient | Metronidazole |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 250mg; 375mg; 500mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 2 of 24 | |
|---|---|
| Drug Name | Flagyl er |
| PubMed Health | Metronidazole (On the skin) |
| Drug Classes | Antiacne Antibacterial |
| Drug Label | FLAGYL metronidazole extended release tablets is an oral formulation of the synthetic nitroimidazole antimicrobial agent, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural formula:FLAGYL (metronidazole) extended release tabl... |
| Active Ingredient | Metronidazole |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 750mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 3 of 24 | |
|---|---|
| Drug Name | Flagyl i.v. rtu in plastic container |
| PubMed Health | Metronidazole |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antiacne Antibacterial, Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori |
| Drug Label | METROCREAM Topical Cream contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75%) in an emollient cream consisting of benzyl alcohol, emulsifying wax, glycerin, isopropyl palmitate, purified water, sorbitol solution, lactic acid a... |
| Active Ingredient | Metronidazole |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/100ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare; Pfizer |
| 4 of 24 | |
|---|---|
| Drug Name | Metro i.v. in plastic container |
| PubMed Health | Metronidazole (On the skin) |
| Drug Classes | Antiacne Antibacterial |
| Drug Label | METROGEL-VAGINAL is the intravaginal dosage form of the synthetic antibacterial agent, metronidazole, USP at a concentration of 0.75%. Metronidazole is a member of the imidazole class of antibacterial agents and is classified therapeutically as an an... |
| Active Ingredient | Metronidazole |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/100ml |
| Market Status | Prescription |
| Company | B Braun |
| 5 of 24 | |
|---|---|
| Drug Name | Metrocream |
| PubMed Health | Metronidazole |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antiacne Antibacterial, Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori |
| Drug Label | METROGEL-VAGINAL is the intravaginal dosage form of the synthetic antibacterial agent, metronidazole, USP at a concentration of 0.75%. Metronidazole is a member of the imidazole class of antibacterial agents and is classified therapeutically as an an... |
| Active Ingredient | Metronidazole |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 6 of 24 | |
|---|---|
| Drug Name | Metrogel |
| Drug Label | MetroLotion (metronidazole lotion) Topical Lotion contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75% w/w) in a lotion consisting of benzyl alcohol, carbomer 941, cyclomethicone, glycerin, glyceryl stearate, light mineral oil,... |
| Active Ingredient | Metronidazole |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 1%; 0.75% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 7 of 24 | |
|---|---|
| Drug Name | Metrogel-vaginal |
| Drug Label | Metronidazole is an oral synthetic antiprotozoal and antibacterial agent, 1-(which has the following structural formula:Metronidazole 250 mg and 500 mg tablets, for oral administration, contain the inactive ingredients: colloidal silicon dioxide, hyd... |
| Active Ingredient | Metronidazole |
| Dosage Form | Gel |
| Route | Vaginal |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Medicis |
| 8 of 24 | |
|---|---|
| Drug Name | Metrolotion |
| Drug Label | NORITATE (metronidazole cream) Cream, 1%, contains metronidazole, USP. Chemically, metronidazole is 2-methyl-5-nitro-1H-imidazole-1-ethanol. The molecular formula for metronidazole is C6H9N3O3. It has the following structural formula:Metronidazole ha... |
| Active Ingredient | Metronidazole |
| Dosage Form | Lotion |
| Route | Topical |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 9 of 24 | |
|---|---|
| Drug Name | Metronidazole |
| Drug Label | VANDAZOLE (metronidazole vaginal gel, 0.75%) is the vaginal dosage form of the nitroimidazole antimicrobial metronidazole at a concentration of 0.75%. Chemically, metronidazole is a 2-methyl-5-nitroimidazole-1-ethanol. C6H9N3O3 M.W. 171.16VANDAZOLE... |
| Active Ingredient | Metronidazole |
| Dosage Form | Tablet, extended release; Tablet; Cream; Capsule; Lotion; Gel |
| Route | Oral; Topical; Vaginal |
| Strength | 250mg; 1%; 375mg; 500mg; 0.75%; 750mg; 1.3% |
| Market Status | Prescription |
| Company | Alembic; Alembic Pharms; Teva Pharms Usa; Taro; Par Pharm; Watson Labs; Fougera Pharms; Mutual Pharm; Tolmar; Pliva; G And W Labs; Unichem Labs |
| 10 of 24 | |
|---|---|
| Drug Name | Metronidazole in plastic container |
| Active Ingredient | Metronidazole |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/100ml |
| Market Status | Prescription |
| Company | Hospira; Claris Lifesciences |
| 11 of 24 | |
|---|---|
| Drug Name | Noritate |
| Active Ingredient | Metronidazole |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 12 of 24 | |
|---|---|
| Drug Name | Vandazole |
| Active Ingredient | Metronidazole |
| Dosage Form | Gel |
| Route | Vaginal |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Teva Pharms |
| 13 of 24 | |
|---|---|
| Drug Name | Flagyl |
| PubMed Health | Metronidazole |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antiacne Antibacterial, Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori |
| Drug Label | FLAGYL (metronidazole) tablets, 250 mg or 500 mg is an oral formulation of the synthetic nitroimidazole antimicrobial, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural formula:FLAGYL (metronidazole) tablets contain 250 mg o... |
| Active Ingredient | Metronidazole |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 250mg; 375mg; 500mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 14 of 24 | |
|---|---|
| Drug Name | Flagyl er |
| PubMed Health | Metronidazole (On the skin) |
| Drug Classes | Antiacne Antibacterial |
| Drug Label | FLAGYL metronidazole extended release tablets is an oral formulation of the synthetic nitroimidazole antimicrobial agent, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural formula:FLAGYL (metronidazole) extended release tabl... |
| Active Ingredient | Metronidazole |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 750mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 15 of 24 | |
|---|---|
| Drug Name | Flagyl i.v. rtu in plastic container |
| PubMed Health | Metronidazole |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antiacne Antibacterial, Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori |
| Drug Label | METROCREAM Topical Cream contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75%) in an emollient cream consisting of benzyl alcohol, emulsifying wax, glycerin, isopropyl palmitate, purified water, sorbitol solution, lactic acid a... |
| Active Ingredient | Metronidazole |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/100ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare; Pfizer |
| 16 of 24 | |
|---|---|
| Drug Name | Metro i.v. in plastic container |
| PubMed Health | Metronidazole (On the skin) |
| Drug Classes | Antiacne Antibacterial |
| Drug Label | METROGEL-VAGINAL is the intravaginal dosage form of the synthetic antibacterial agent, metronidazole, USP at a concentration of 0.75%. Metronidazole is a member of the imidazole class of antibacterial agents and is classified therapeutically as an an... |
| Active Ingredient | Metronidazole |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/100ml |
| Market Status | Prescription |
| Company | B Braun |
| 17 of 24 | |
|---|---|
| Drug Name | Metrocream |
| PubMed Health | Metronidazole |
| Drug Classes | Amebicide, Extraintestinal, Amebicide, Intestinal, Antiacne Antibacterial, Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori |
| Drug Label | METROGEL-VAGINAL is the intravaginal dosage form of the synthetic antibacterial agent, metronidazole, USP at a concentration of 0.75%. Metronidazole is a member of the imidazole class of antibacterial agents and is classified therapeutically as an an... |
| Active Ingredient | Metronidazole |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 18 of 24 | |
|---|---|
| Drug Name | Metrogel |
| Drug Label | MetroLotion (metronidazole lotion) Topical Lotion contains metronidazole, USP, at a concentration of 7.5 mg per gram (0.75% w/w) in a lotion consisting of benzyl alcohol, carbomer 941, cyclomethicone, glycerin, glyceryl stearate, light mineral oil,... |
| Active Ingredient | Metronidazole |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 1%; 0.75% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 19 of 24 | |
|---|---|
| Drug Name | Metrogel-vaginal |
| Drug Label | Metronidazole is an oral synthetic antiprotozoal and antibacterial agent, 1-(which has the following structural formula:Metronidazole 250 mg and 500 mg tablets, for oral administration, contain the inactive ingredients: colloidal silicon dioxide, hyd... |
| Active Ingredient | Metronidazole |
| Dosage Form | Gel |
| Route | Vaginal |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Medicis |
| 20 of 24 | |
|---|---|
| Drug Name | Metrolotion |
| Drug Label | NORITATE (metronidazole cream) Cream, 1%, contains metronidazole, USP. Chemically, metronidazole is 2-methyl-5-nitro-1H-imidazole-1-ethanol. The molecular formula for metronidazole is C6H9N3O3. It has the following structural formula:Metronidazole ha... |
| Active Ingredient | Metronidazole |
| Dosage Form | Lotion |
| Route | Topical |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 21 of 24 | |
|---|---|
| Drug Name | Metronidazole |
| Drug Label | VANDAZOLE (metronidazole vaginal gel, 0.75%) is the vaginal dosage form of the nitroimidazole antimicrobial metronidazole at a concentration of 0.75%. Chemically, metronidazole is a 2-methyl-5-nitroimidazole-1-ethanol. C6H9N3O3 M.W. 171.16VANDAZOLE... |
| Active Ingredient | Metronidazole |
| Dosage Form | Tablet, extended release; Tablet; Cream; Capsule; Lotion; Gel |
| Route | Oral; Topical; Vaginal |
| Strength | 250mg; 1%; 375mg; 500mg; 0.75%; 750mg; 1.3% |
| Market Status | Prescription |
| Company | Alembic; Alembic Pharms; Teva Pharms Usa; Taro; Par Pharm; Watson Labs; Fougera Pharms; Mutual Pharm; Tolmar; Pliva; G And W Labs; Unichem Labs |
| 22 of 24 | |
|---|---|
| Drug Name | Metronidazole in plastic container |
| Active Ingredient | Metronidazole |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/100ml |
| Market Status | Prescription |
| Company | Hospira; Claris Lifesciences |
| 23 of 24 | |
|---|---|
| Drug Name | Noritate |
| Active Ingredient | Metronidazole |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 24 of 24 | |
|---|---|
| Drug Name | Vandazole |
| Active Ingredient | Metronidazole |
| Dosage Form | Gel |
| Route | Vaginal |
| Strength | 0.75% |
| Market Status | Prescription |
| Company | Teva Pharms |
Mesh Heading: Anti-infective agents, antiprotozoal agents, radiation-sensitizing agents
National Library of Medicine, SIS; ChemIDplus Record for <
MEDICATION (VET): Antiprotozoal (Trichomonas); antiamebic; antibacterial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1097
MEDICATION (VET): The success of metronidazole in treating human infections of giardiasis, vaginal and oral trichomoniasis, and hepatic and intestinal amoebiasis has lead to investigation of its potential use against certain protozoan diseases of domestic animals. These are principally bovine urogenital trichomoniasis and canine, feline, or primate intestinal giardiasis, trichomoniasis, amoebiasis, or Balantidium infection. ...
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 885
Oral metronidazole (extended release formulation) is used in the treatment of bacterial vaginosis caused by Gardnerella vaginalis, Mobiluncus spp, mycoplasma hominis and anaerobes (peptostreptococcus spp and Bacteroides spp). /Included in US or Canadian product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2069
For more Therapeutic Uses (Complete) data for METRONIDAZOLE (25 total), please visit the HSDB record page.
Metronidazole crosses the placenta and enters the fetal circulation rapidly. Adequate and well-controlled studies in humans have not been done. ... However, the use of metronidazole in the treatment of trichomoniasis is not recommended during the first trimester. If metronidazole is used during the second and the third trimesters for trichomoniasis it is recommended that its use be limited to those patients whose symptoms are not controlled by local palliative treatment. Also, the 1 day course of therapy should not be used since this results in higher maternal and fetal serum concentrations.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2070
No information is available on the relationship of age to the effects of metronidazole in geriatric patients. However, elderly patients are more likely to have an age-related decrease in hepatic function, which may require an adjustment in dosage in patients receiving metronidazole.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2070
Peripheral neuropathy, characterized by numbness, tingling, or paresthesia of an extremity, and convulsive seizures have been reported rarely with oral or IV metronidazole. Peripheral neuropathy is usually reversible if metronidazole is discontinued but may persist in patients who receive prolonged therapy or higher than recommended dosage of the drug. Dizziness, vertigo, incoordination, ataxia, confusion, irritability, depression, weakness, insomnia, headache, syncope, tinnitus, and hearing loss have also occurred with metronidazole. Headache occurred in 18% of nonpregnant women receiving oral metronidazole (administered as extended-release tablets) for bacterial vaginosis, and among those reporting headache, 10% described it as severe.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 889
Urethral burning or discomfort, dysuria, cystitis, polyuria, incontinence, a sense of pelvic pressure, dryness of the vagina or vulva, dyspareunia, and decreased libido have been reported with oral metronidazole. Urine may be dark or reddish-brown in color following oral or IV administration of metronidazole due to the presence of water-soluble pigments which result from metabolism of the drug. Vulvovaginal candidiasis (or yeast vaginitis) was reported in 15% of nonpregnant women receiving oral metronidazole (administered as extended-release tablets) and in 12% of those receiving clindamycin phosphate (2% clindamycin) vaginal cream in a comparative study for the treatment of bacterial vaginosis. Although a definite causal relationship to the drug has not been established, genital pruritus, dysmenorrhea, and urinary tract infection have been reported in 5, 3, and 2%, respectively, of nonpregnant women receiving oral metronidazole (administered as extended-release tablets) for the treatment of bacterial vaginosis.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 890
For more Drug Warnings (Complete) data for METRONIDAZOLE (18 total), please visit the HSDB record page.
Metronidazole is indicated for the treatment of confirmed trichomoniasis caused by Trichomonas vaginalis (except for in the first trimester of pregnancy) and the patient's sexual partners, bacterial vaginosis, certain types of amebiasis, and various anaerobic infections. The above anaerobic infections may occur on the skin and skin structures, the abdomen, the heart, reproductive organs, central nervous system, and the respiratory system. Some may also be present in the bloodstream in cases of septicemia. Common infections treated by metronidazole are Bacteroides species infections, Clostridium infections, and Fusobacterium infections, as well as Peptococcus and Peptostreptococcus infections. It is also used off-label in the treatment of Crohn's disease and rosacea, as a prophylactic agent after surgery, and in the treatment of Helicobacter pylori infection. It has also been studied in the prevention of preterm births and to treat periodontal disease.
Treatment of Helicobacter spp. infections
Metronidazole treats amebiasis, trichomoniasis, and giardiasis, exerting both antibacterial and antiprotozoal activities. Metronidazole is an effective treatment for some anaerobic bacterial infections. Metronidazole has shown antibacterial activity against the majority of obligate anaerobes, however, during in vitro studies, it does not demonstrate significant action against facultative anaerobes or obligate aerobes. The nitro group reduction of metronidazole by anaerobic organisms is likely responsible for the drug's antimicrobial cytotoxic effects, causing DNA strand damage to microbes. A note on convulsions and neuropathy and carcinogenesis It is important to be aware of the risk of peripheral neuropathy and convulsions associated with metronidazole, especially at higher doses. If convulsions or numbness of an extremity occur, discontinue the drug immediately. Metronidazole has been found to be carcinogenic in mice and rats. The relevance to this effect in humans is unknown. It is advisable to only administer metronidazole when clinically necessary and only for its approved indications.
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
J01XD01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AB - Antiinfectives and antiseptics for local oral treatment
A01AB17 - Metronidazole
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BX - Other chemotherapeutics
D06BX01 - Metronidazole
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AF - Imidazole derivatives
G01AF01 - Metronidazole
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XD - Imidazole derivatives
J01XD01 - Metronidazole
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01A - Agents against amoebiasis and other protozoal diseases
P01AB - Nitroimidazole derivatives
P01AB01 - Metronidazole
Absorption
After the intravenous infusion of a 1.5g dose, peak concentration was reached within 1 hour and was peak level of 30-40 mg/L. When a multiple-dose regimen of 500mg three times a day administered intravenously, steady-state concentrations were achieved within about 3 days and peak concentration was measured at 26 mg/L. When administered orally in the tablet form, metronidazole is absorbed entirely absorbed, showing a bioavailability of greater than 90%. One resource indicates that Cmax after a single oral dose of 500mg metronidazole ranges from 8 to 13 mg/L, with a Tmax of 25 minutes to 4 hours. The AUC following a single 500mg oral dose of metronidazole was 122 10.3 mg/L h. A note on the absorption of topical preparations Insignificant percutaneous absorption of metronidazole occurs after the application of 1% metronidazole cream topically. Healthy volunteers applied one 100 mg dose of 14C-labelled metronidazole 2% cream to unbroken skin. After 12 hours, metronidazole was not detected in the plasma. Approximately 0.1% to 1% of the administered metronidazole was measured in the urine and feces.
Route of Elimination
Metronidazole and metabolites are 60 to 80% eliminated in the urine, and 6-15% excreted in the feces.
Volume of Distribution
Metronidazole is widely distributed throughout the body and various body fluids. They include the bile, saliva, breastmilk, cerebrospinal fluid, and the placenta. Steady-state volume distribution of metronidazole in adults ranges from 0.51 to 1.1 L/kg. It attains 60 to 100% of plasma concentrations in various tissues, such as the central nervous system, however, is not measured in high concentrations in the placental tissue.
Clearance
Dose adjustments may be required in patients with hepatic impairment, as clearance is impaired in these patients. The clearance of metronidazole in the kidneys is estimated at 10 mL/min/1.73 m2. The total clearance from serum is about 2.1 to 6.4 L/h/kg.
Well absorbed orally; bioavailability at least 80%.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2069
Distributed to saliva, bile, seminal fluid, breast milk, bone, liver and liver abscesses, lungs, and vaginal secretions; crosses the placenta and blood-brain barrier, also.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2069
At least 80% of an oral dose of metronidazole is absorbed from the GI tract. Following oral administration of a single 250-mg, 500-mg, or 2-g dose of metronidazole as immediate-release (conventional) preparations in healthy, fasting adults, peak plasma concentrations of unchanged drug and active metabolites are attained within 1-3 hours and average 4.6-6.5 ug/mL, 11.5-13 ug/mL, and 30-45 ug/mL, respectively. When a single 750-mg dose of metronidazole is administered as two 375-mg capsules or three 250-mg conventional tablets in healthy, fasting adult women, average peak plasma concentrations of unchanged drug and active metabolites of 20.4-21.4 ug/mL are attained in an average of 1.4-1.6 hours; metronidazole capsules and conventional tablets are bioequivalent at a single dose of 750 mg. The rate of absorption and peak plasma concentrations of metronidazole are decreased when conventional tablets or capsules of the drug are administered with food; however, the total amount of drug absorbed is not affected.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 892
Following oral administration of metronidazole 750 mg once daily as the extended-release tablet for 7 consecutive days in healthy, adult women, steady-state peak plasma concentrations average 12.5 mcg/mL and are attained an average of 6.8 hours after the dose when the drug is given under fasting conditions; when the drug is given at the same dosage under nonfasting conditions, steady-state peak plasma concentrations average 19.4 mcg/mL and are attained an average of 4.6 hours after the dose. Administration of metronidazole extended-release tablets with food increases the rate of absorption and peak plasma concentrations of the drug. According to the manufacturer, metronidazole extended-release and conventional tablets are bioequivalent at a dose of 750 mg given under fasting conditions.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 892
For more Absorption, Distribution and Excretion (Complete) data for METRONIDAZOLE (12 total), please visit the HSDB record page.
Metronidazole undergoes hepatic metabolism via hydroxylation, oxidation, and glucuronidation. The metabolism of metronidazole yields 5 metabolites. The hydroxy metabolite, 1-(2-hydroxy-ethyl)-2-hydroxy methyl-5-nitroimidazole, is considered the major active metabolite. Unchanged metronidazole is found in the plasma along with small amounts of its 2- hydroxymethyl metabolite. Several metabolites of metronidazole are found in the urine. They are primarily a product of side-chain oxidation in addition to glucuronide conjugation. Only 20% of the dose found in the urine is accounted for by unchanged metronidazole. The two main oxidative metabolites of metronidazole are hydroxy and acetic acid metabolites.
Approximately 30-60% of an oral or IV dose of metronidazole is metabolized in the liver by hydroxylation, side-chain oxidation, and glucuronide conjugation. The major metabolite, 2-hydroxy metronidazole, has some antibacterial and antiprotozoal activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 892
... Four other nitro-group-containing metabolites have been identified, each derived from side-chain oxidation of ethyl and/or methyl group. They include 1-acetic acid-2-methyl-5-nitroimidazole and 1-(2-hydroxyethyl)-2-carboxylic acid-5-nitroimidazole salt.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 118 (1977)
The liver is the main site of metabolism, and this accounts for over 50% of the systemic clearance of metronidazole. The 2 principal metabolites result from oxidation of side chains, a hydroxy derivative and an acid. The hydroxy metabolite has a longer half-life (about 12 hr) and nearly 50% of the antitrichomonal activity of metronidazole. Formation of glucuronides also is observed. Small quantities of reduced metabolites, including ring-cleavage products, are formed by the gut flora. The urine of some patients may be reddish-brown owing to the presence of unidentified pigments derived from the drug.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1106
The elimination half-life of metronidazole is 7.3 1.0 after a single 500mg IV dose in healthy subjects. Another resource indicates that the elimination half-life for metronidazole ranges from 6 to 10 hours.
The plasma half-life of metronidazole is reported to be 6-8 hours in adults with normal renal and hepatic function. In one study using radiolabeled metronidazole hydrochloride, the half-life of unchanged metronidazole averaged 7.7 hours and the half-life of total radioactivity averaged 11.9 hours. The plasma half-life of metronidazole is not affected by changes in renal function; however, the half-life may be prolonged in patients with impaired hepatic function. In one study in adults with alcoholic liver disease and impaired hepatic function, half-life of metronidazole averaged 18.3 hours (range: 10.3-29.5 hours).
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 892
Half-life: Neonates 25-75 hours; Others: 6-8 hours, increases with hepatic impairment.
Lelkin, J.B., Paloucek, F.P., Poisoning & Toxicology Compendium. LEXI-COMP Inc. & American Pharmaceutical Association, Hudson, OH 1998., p. 390
The elimination half-life in dogs is 4.5hr, and in horses 1.5-3.3hr
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2098
The exact mechanism of action of metronidazole has not been fully established, however, it is possible that an intermediate in the reduction of metronidazole which is only made by anaerobic bacteria and protozoa, binds deoxyribonucleic acid and electron-transport proteins of organisms, blocking nucleic acid synthesis. After administration, metronidazole enters cells by passive diffusion. Following this, ferredoxin or flavodoxin reduce its nitro group to nitro radicals. The redox potential of the electron transport portions of anaerobic or microaerophilic microorganisms renders metronidazole selective to these organisms, which cause nitro group reduction, leading to the production of toxic metabolites. These include N-(2-hydroxyethyl) oxamic acid and acetamide, which may damage DNA of replicating organisms.
Microbicidal; active against most obligate anaerobic bacteria and protozoa by undergoing intracellular chemical reduction via mechanisms unique to anaerobic metabolism. Reduced metronidazole, which is cytotoxic but short-lived, interacts with DNA to cause loss of helical structure, strand breakage, and resultant inhibition of nucleic acid synthesis and cell death.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2069
Metronidazole is bactericidal, amebicidal, and trichomonacidal in action. The exact mechanism of action of the drug has not been fully elucidated. Metronidazole is un-ionized at physiologic pH and is readily taken up by anaerobic organisms or cells. In susceptible organisms or cells, metronidazole is reduced by low-redox-potential electron transport proteins (e.g., nitroreductases such as ferredoxin) to unidentified polar product(s) which lack the nitro group. The reduction product(s) appears to be responsible for the cytotoxic and antimicrobial effects of the drug which include disruption of DNA and inhibition of nucleic acid synthesis. Metronidazole is equally effective against dividing and nondividing cells.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 891
In in vivo studies in rats given metronidazole in dosages of 2-4 mg/100 g of body weight, the drug reportedly inhibited the development of formalin-induced edema in the rat paw. In vitro in neutrophils, metronidazole has a dose-dependent inhibitory effect on generation of hydrogen peroxide and hydroxyl radicals, oxidants that may cause tissue injury at the site of inflammation. This antioxidant effect appears to be caused by a direct effect on neutrophil function and may contribute to the drug's anti-inflammatory effect in vivo.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 891
Results of in vitro studies using leukocytes obtained from patients with Crohn's disease indicate that exposing the cells to metronidazole concentrations of 10 or 50 mcg/mL improved both spontaneous and induced leukocyte migration in cells that previously exhibited reduced migration; the drug had no effect on leukocytes obtained from healthy adults or patients with Crohn's disease when the cells exhibited normal migration prior to exposure to the drug. This effect on leukocyte migration also was observed in vivo in adults with Crohn's disease who received a single 400-mg dose of metronidazole. It has been suggested that metronidazole may increase leukocyte migration by a direct effect on the leukocytes, possibly by causing the release of surface-bound immune complexes from the cell surface.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 891

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
66
PharmaCompass offers a list of Metronidazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Metronidazole manufacturer or Metronidazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Metronidazole manufacturer or Metronidazole supplier.
PharmaCompass also assists you with knowing the Metronidazole API Price utilized in the formulation of products. Metronidazole API Price is not always fixed or binding as the Metronidazole Price is obtained through a variety of data sources. The Metronidazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Metronidazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Metronidazole, including repackagers and relabelers. The FDA regulates Metronidazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Metronidazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Metronidazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Metronidazole supplier is an individual or a company that provides Metronidazole active pharmaceutical ingredient (API) or Metronidazole finished formulations upon request. The Metronidazole suppliers may include Metronidazole API manufacturers, exporters, distributors and traders.
click here to find a list of Metronidazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Metronidazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Metronidazole active pharmaceutical ingredient (API) in detail. Different forms of Metronidazole DMFs exist exist since differing nations have different regulations, such as Metronidazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Metronidazole DMF submitted to regulatory agencies in the US is known as a USDMF. Metronidazole USDMF includes data on Metronidazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Metronidazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Metronidazole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Metronidazole Drug Master File in Japan (Metronidazole JDMF) empowers Metronidazole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Metronidazole JDMF during the approval evaluation for pharmaceutical products. At the time of Metronidazole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Metronidazole suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Metronidazole Drug Master File in Korea (Metronidazole KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Metronidazole. The MFDS reviews the Metronidazole KDMF as part of the drug registration process and uses the information provided in the Metronidazole KDMF to evaluate the safety and efficacy of the drug.
After submitting a Metronidazole KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Metronidazole API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Metronidazole suppliers with KDMF on PharmaCompass.
A Metronidazole CEP of the European Pharmacopoeia monograph is often referred to as a Metronidazole Certificate of Suitability (COS). The purpose of a Metronidazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Metronidazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Metronidazole to their clients by showing that a Metronidazole CEP has been issued for it. The manufacturer submits a Metronidazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Metronidazole CEP holder for the record. Additionally, the data presented in the Metronidazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Metronidazole DMF.
A Metronidazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Metronidazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Metronidazole suppliers with CEP (COS) on PharmaCompass.
A Metronidazole written confirmation (Metronidazole WC) is an official document issued by a regulatory agency to a Metronidazole manufacturer, verifying that the manufacturing facility of a Metronidazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Metronidazole APIs or Metronidazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Metronidazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Metronidazole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Metronidazole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Metronidazole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Metronidazole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Metronidazole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Metronidazole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Metronidazole suppliers with NDC on PharmaCompass.
Metronidazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Metronidazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Metronidazole GMP manufacturer or Metronidazole GMP API supplier for your needs.
A Metronidazole CoA (Certificate of Analysis) is a formal document that attests to Metronidazole's compliance with Metronidazole specifications and serves as a tool for batch-level quality control.
Metronidazole CoA mostly includes findings from lab analyses of a specific batch. For each Metronidazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Metronidazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Metronidazole EP), Metronidazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Metronidazole USP).
