
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Mtopirone
2. Methbipyranone
3. Methopyrapone
4. Metopiron
5. Metopirone
6. Su 4885
1. 54-36-4
2. 2-methyl-1,2-di-3-pyridyl-1-propanone
3. Metopirone
4. Methopyrapone
5. Metopiron
6. Methapyrapone
7. Methopirapone
8. Methopyrinine
9. Methopyrone
10. Metopyrone
11. Metyrapon
12. Methbipyranone
13. Mepyrapone
14. Metroprione
15. Metapirone
16. Metapyron
17. Metirapona
18. Metyraponum
19. 2-methyl-1,2-dipyridin-3-ylpropan-1-one
20. Su 4885
21. 1-propanone, 2-methyl-1,2-di-3-pyridinyl-
22. 2-methyl-1,2-di(pyridin-3-yl)propan-1-one
23. 2-methyl-1,2-di-3-pyridinyl-1-propanone
24. 2-methyl-1,2-bis(3-pyridyl)-1-propanone
25. Nsc 25265
26. Nsc-25265
27. 1-propanone, 2-methyl-1,2-di-3-pyridyl-
28. Chembl934
29. Zs9kd92h6v
30. Chebi:44241
31. 2-methyl-1,2-bis(pyridin-3-yl)propan-1-one
32. Cas-54-36-4
33. Ncgc00016242-01
34. Dsstox_cid_3314
35. Dsstox_rid_76971
36. Dsstox_gsid_23314
37. Metyraponum [inn-latin]
38. Metirapona [inn-spanish]
39. Myt
40. Smr000059134
41. 1,2-di-3-pyridyl-2-methyl-1-propanone
42. Hsdb 2500
43. Metopirone (tn)
44. Einecs 200-206-2
45. Unii-zs9kd92h6v
46. 1-propanone, 1,2-di-3-pyridyl-2-methyl-
47. Metyrapone [usan:usp:inn:ban:jan]
48. Metyrapone [mi]
49. Metyrapone [inn]
50. Metyrapone [jan]
51. 2-methyl-1,2-bis(3-pyridyl)propan-1-one
52. Prestwick0_000904
53. Prestwick1_000904
54. Prestwick2_000904
55. Prestwick3_000904
56. Metyrapone [hsdb]
57. Metyrapone [usan]
58. 2-methyl-1,2-dipyridin-3-yl-propan-1-one
59. Metyrapone [vandf]
60. Metyrapone [mart.]
61. Metyrapone [usp-rs]
62. Metyrapone [who-dd]
63. Bidd:pxr0082
64. Bspbio_000748
65. Mls001066377
66. Mls001335881
67. Mls001335882
68. Schembl637432
69. Spbio_002947
70. 1-propanone,2-di-3-pyridyl-
71. Amy832
72. Bpbio1_000824
73. Gtpl5224
74. Metyrapone (jp17/usp/inn)
75. Zinc1728
76. Dtxsid1023314
77. Metyrapone [orange Book]
78. 1-propanone,2-di-3-pyridinyl-
79. Hms1570f10
80. Hms2094i07
81. Hms2097f10
82. Hms2235e16
83. Hms3259o05
84. Hms3371b16
85. Hms3714f10
86. Hms3742e21
87. Hms3869k13
88. Metyrapone [usp Monograph]
89. Pharmakon1600-01506014
90. Bcp19099
91. Ex-a1403
92. Hy-b1232
93. Nsc25265
94. Tox21_110323
95. Bdbm50028166
96. Nsc760076
97. S5416
98. Akos015919707
99. Metyrapone, >=98% (hplc), Solid
100. Tox21_110323_1
101. Ccg-213598
102. Cs-4879
103. Db01011
104. Nc00462
105. Nsc-760076
106. Ncgc00016242-02
107. Ncgc00016242-03
108. Ncgc00016242-05
109. Ncgc00161837-01
110. As-56195
111. Db-052546
112. Ab00513955
113. B7347
114. Eu-0009322
115. Ft-0603230
116. Su-4885; Su 4885; Su4885
117. 2-methyl-1,2-di(3-pyridinyl)-1-propanone #
118. C07205
119. D00410
120. D70339
121. 2-methyl-1,2-di-3-pyridyl-1-propanone, 96%
122. Ab00513955_07
123. 054m364
124. A830124
125. Q821641
126. Sr-01000765398
127. Sr-01000765398-2
128. Brd-k46862739-001-03-6
129. Metyrapone, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 226.27 g/mol |
|---|---|
| Molecular Formula | C14H14N2O |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 226.110613074 g/mol |
| Monoisotopic Mass | 226.110613074 g/mol |
| Topological Polar Surface Area | 42.8 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 275 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Metopirone |
| PubMed Health | Metyrapone (By mouth) |
| Drug Classes | Diagnostic Agent, Pituitary Function |
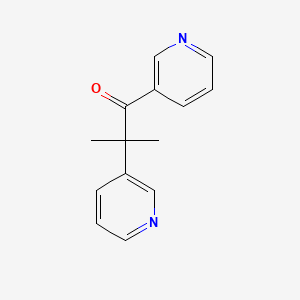
| Drug Label | Metopirone, metyrapone USP, is an inhibitor of endogenous adrenal corticosteriod synthesis, available as 250-mg capsules for oral administration. Its chemical name is 2-methyl-1, 2-di-3-pyridyl-1-propanone, and its structural formula isMetopirone s... |
| Active Ingredient | Metyrapone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Hra Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Metopirone |
| PubMed Health | Metyrapone (By mouth) |
| Drug Classes | Diagnostic Agent, Pituitary Function |
| Drug Label | Metopirone, metyrapone USP, is an inhibitor of endogenous adrenal corticosteriod synthesis, available as 250-mg capsules for oral administration. Its chemical name is 2-methyl-1, 2-di-3-pyridyl-1-propanone, and its structural formula isMetopirone s... |
| Active Ingredient | Metyrapone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 250mg |
| Market Status | Prescription |
| Company | Hra Pharma |
METYRAPONE IS USED TO TEST ABILITY OF PITUITARY TO RESPOND TO DECR CONCN OF PLASMA CORTISOL. ADMIN...TO PT WITH DISEASE OF HYPOTHALAMICO-PITUITARY COMPLEX.../IS/ NOT FOLLOWED BY INCR RENAL EXCRETION OF "17-HYDROXYCORTICOIDS."
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1502
.../METYRAPONE HAS/ BEEN USED WITH SPIRONOLACTONE & PREDNISONE TO RELIEVE SEVERE EDEMA, BUT...HAS BEEN REPLACED BY MORE POTENT DIURETICS. .../ALSO/ USED INVESTIGATIONALLY TO LOWER PLASMA CHOLESTEROL LEVELS IN PT WITH FAMILIAL HYPERCHOLESTEROLEMIA TYPE II, BUT WELL-CONTROLLED CLINICAL STUDIES...NOT YET AVAILABLE.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 36:66
METYRAPONE MAY BE USED TO CONFIRM DEXAMETHASONE SUPPRESSION TEST RESULTS IN DIFFERENTIAL DIAGNOSIS OF ADRENAL HYPERPLASIA & ADRENAL ADENOMA...RESULTS OF THIS TEST MUST BE INTERPRETED CAUTIOUSLY.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 36:66
METYRAPONE HAS BEEN USED EXPTL TO TREAT HYPERCORTISOLISM THAT RESULTS FROM ADRENAL NEOPLASMS THAT FUNCTION AUTONOMOUSLY & FROM ECTOPIC ACTH PRODUCTION BY TUMORS. IT IS NOT SUITABLE FOR CORRECTING EXCESSIVE CORTISOL SECRETION OF CUSHING'S SYNDROME CAUSED BY HYPERSECRETION OF PITUITARY ACTH.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1502
...ADMIN OF METYRAPONE CAN BE USED AS TEST FOR NORMAL HYPOTHALAMICO-PITUITARY FUNCTION ONLY IF ADRENAL GLANDS ARE CAPABLE OF RESPONDING TO ACTH...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1502
IN HIGH DOSES, METYRAPONE MAY INHIBIT 18- & 19-HYDROXYLASE ENZYMES, THUS INHIBITING SYNTHESIS OF OTHER STEROIDS, INCL ESTROGENS. ...DRUG MAY ALSO DECR PLASMA HALF-LIFE OF CORTISOL, INCR GROWTH HORMONE RELEASE, & INDUCE HYPERGLYCEMIA.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 36:66
...DRUG MAY INDUCE ACUTE ADRENAL INSUFFICIENCY IN PT WITH REDUCED ADRENAL SECRETORY CAPACITY. METYRAPONE ALSO INHIBITS SYNTHESIS OF ALDOSTERONE... HOWEVER.../IT/ DOES NOT TYPICALLY CAUSE DEFICIENCY IN MINERALOCORTICOIDS, WITH CONSEQUENT LOSS OF SODIUM & RETENTION OF POTASSIUM...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1502
OCCASIONALLY, ARTERIAL BLOOD PRESSURE MAY FALL & MODERATE INCR IN PULSE RATE MAY INSUE. ...SHOULD NOT BE USED IN PT WITH ADRENOCORTICAL INSUFFICIENCY...SAFE USE OF DRUG IN PREGNANCY HAS NOT BEEN ESTABLISHED.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 36:66
ABILITY OF ADRENAL TO RESPOND TO ACTH SHOULD BE DEMONSTRATED BEFORE METYRAPONE IS EMPLOYED...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1502
For more Drug Warnings (Complete) data for METYRAPONE (8 total), please visit the HSDB record page.
Used as a diagnostic drug for testing hypothalamic-pituitary ACTH function. Occasionally used in Cushing's syndrome.
Metopirone is an inhibitor of endogenous adrenal corticosteroid synthesis.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
V - Various
V04 - Diagnostic agents
V04C - Other diagnostic agents
V04CD - Tests for pituitary function
V04CD01 - Metyrapone
Absorption
Absorbed rapidly and well when administered orally. Peak plasma concentrations are usually reached 1 hour after administration.
Route of Elimination
After administration of 4.5 g metyrapone (750 mg every 4 hours), an average of 5.3% of the dose was excreted in the urine in the form of metyrapone (9.2% free and 90.8% as glucuronide) and 38.5% in the form of metyrapol (8.1% free and 91.9% as glucuronide) within 72 hours after the first dose was given.
GASTROINTESTINAL ABSORPTION OF METYRAPONE IS VARIABLE. FOLLOWING ORAL ADMIN OF 750 MG OF METYRAPONE EVERY 4 HR TO NORMAL PT, PEAK PLASMA LEVELS OF ABOUT 1.2 UG M/L...REACHED AFTER THIRD DOSE...0.4 UG/ML...PRESENT IMMEDIATELY AFTER SIXTH DOSE.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 36:66
...PLASMA LEVELS OF METYRAPONE...IN RATS WERE SHOWN TO EXHIBIT A CIRCADIAN PATTERN WHEN ANIMALS WERE KEPT IN ALTERNATING 12-HR LIGHT-12 HR DARK REGIMEN. HALF-LIFE...OBSERVED @ 10:00 PM WAS APPROX 2.5 TIMES LONGER THAN THAT OBSERVED @ 10:00 AM...
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 408
...WITHIN 2 DAYS FOLLOWING ORAL ADMIN OF 750 MG...EVERY 4 HR FOR 6 DOSES, APPROX 0.5%...IS EXCRETED IN URINE UNCHANGED, 3% IS EXCRETED AS REDUCED METABOLITE, & 37% AS GLUCURONIDE CONJUGATES OF METYRAPONE & ITS METABOLITES.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 36:66
Hepatic. The major biotransformation is reduction of the ketone to metyrapol, an active alcohol metabolite. Metyrapone and metyrapol are both conjugated with glucuronide.
/METYRAPONE IS METABOLIZED BY/ ONE ENZYME, PRESENT IN MICROSOMAL FRACTION OF RAT LIVER, IS NADPH-DEPENDENT & IS ACTIVE UNDER AEROBIC CONDITIONS, REDUCING COMPD TO 2-METHYL-1,2-BIS-(3-PYRIDYL)PROPAN-1-OL...SECOND ENZYME...HAS NOT BEEN IDENTIFIED...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 372
1.9 ±0.7 hours.
METYRAPONE HAS PLASMA HALF-LIFE OF ABOUT 20-26 MIN.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 36:66
The pharmacological effect of Metopirone is to reduce cortisol and corticosterone production by inhibiting the 11-ß-hydroxylation reaction in the adrenal cortex. Removal of the strong inhibitory feedback mechanism exerted by cortisol results in an increase in adrenocorticotropic hormone (ACTH) production by the pituitary. With continued blockade of the enzymatic steps leading to production of cortisol and corticosterone, there is a marked increase in adrenocortical secretion of their immediate precursors, 11-desoxycortisol and desoxycorticosterone, which are weak suppressors of ACTH release, and a corresponding elevation of these steroids in the plasma and of their metabolites in the urine. These metabolites are readily determined by measuring urinary 17-hydroxycorticosteroids (17-OHCS) or 17-ketogenic steroids (17-KGS). Because of these actions, metopirone is used as a diagnostic test, with urinary 17-OHCS measured as an index of pituitary ACTH responsiveness. Metopirone may also suppress biosynthesis of aldosterone, resulting in a mild natriuresis.
METYRAPONE REDUCES CORTISOL PRODUCTION BY INHIBITION OF 11BETA-HYDROXYLATION REACTION. BIOSYNTHETIC PROCESS IS TERMINATED @ 11DESOXYCORTISOL... IN NORMAL PERSON...INCR IN ACTH RELEASE FOLLOWS, & SECRETION OF 11DESOXYCORTISOL...ACCELERATED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1502
ORAL ADMIN OF 250 MG/SQ M BODY SURFACE INCR PLASMA CONCN OF GLUCOSE & GROWTH HORMONE IN CHILDREN.
DAMMACO ET AL; EFFECTS OF ORAL ADMIN OF METYRAPONE ON HUMAN GROWTH HORMONE SECRETION & GLUCOSE METABOLISM IN CHILDREN; BOLL SOC ITAL BIOL SPER 50(6) 1315-320 (1974)
@ LOW DOSES PGE2 & PGF2ALPHA RELEASE WERE STIMULATED. @ HIGHER DOSES PGF2ALPHA RELEASE WAS INHIBITED.
PARNHAM MJ; EFFECTS OF METYRAPONE ON RAT UTERINE PROSTAGLANDIN RELEASE & ON SPONTANEOUS SMOOTH MUSCLE ACTIVITY IN VITRO; BR J PHARMACOL 57(3) 465-466 (1976)
 Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.

NDC Package Code : 70219-0001
Start Marketing Date : 2012-07-31
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
NDC Package Code : 70219-0001
Start Marketing Date : 2012-07-31
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
87
PharmaCompass offers a list of Metyrapone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Metyrapone manufacturer or Metyrapone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Metyrapone manufacturer or Metyrapone supplier.
PharmaCompass also assists you with knowing the Metyrapone API Price utilized in the formulation of products. Metyrapone API Price is not always fixed or binding as the Metyrapone Price is obtained through a variety of data sources. The Metyrapone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Metyrapone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Metyrapone, including repackagers and relabelers. The FDA regulates Metyrapone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Metyrapone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Metyrapone supplier is an individual or a company that provides Metyrapone active pharmaceutical ingredient (API) or Metyrapone finished formulations upon request. The Metyrapone suppliers may include Metyrapone API manufacturers, exporters, distributors and traders.
click here to find a list of Metyrapone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Metyrapone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Metyrapone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Metyrapone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Metyrapone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Metyrapone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Metyrapone suppliers with NDC on PharmaCompass.
Metyrapone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Metyrapone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Metyrapone GMP manufacturer or Metyrapone GMP API supplier for your needs.
A Metyrapone CoA (Certificate of Analysis) is a formal document that attests to Metyrapone's compliance with Metyrapone specifications and serves as a tool for batch-level quality control.
Metyrapone CoA mostly includes findings from lab analyses of a specific batch. For each Metyrapone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Metyrapone may be tested according to a variety of international standards, such as European Pharmacopoeia (Metyrapone EP), Metyrapone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Metyrapone USP).
