
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
Australia

0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Fk 463
2. Fk-463
3. Fk463
4. Micafungin
5. Mycamine
1. 208538-73-2
2. Funguard
3. Mycamine
4. Fk463 Sodium
5. Fk-463
6. Micafungin Na
7. Micafungin Sodium Salt
8. Fk 463
9. Micafungin Sodium [usan]
10. Is1up79r56
11. Chebi:80105
12. Fk463
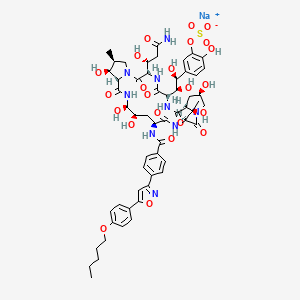
13. Sodium;[5-[(1s,2s)-2-[(3s,6s,9s,11r,15s,18s,20r,21r,24s,25s,26s)-3-[(1r)-3-amino-1-hydroxy-3-oxopropyl]-11,20,21,25-tetrahydroxy-15-[(1r)-1-hydroxyethyl]-26-methyl-2,5,8,14,17,23-hexaoxo-18-[[4-[5-(4-pentoxyphenyl)-1,2-oxazol-3-yl]benzoyl]amino]-1,4,7,13,16,22-hexazatricyclo[22.3.0.09,13]heptacosan-6-yl]-1,2-dihydroxyethyl]-2-hydroxyphenyl] Sulfate
14. Mycamine Sodium
15. Funguard (tn)
16. Mycamine (tn)
17. Mcfg
18. Micafungin Sodium- Bio-x
19. Unii-is1up79r56
20. Micafungin Na [vandf]
21. Micafungin Sodium (jan/usan)
22. Micafungin Sodium [jan]
23. Chembl1237070
24. Ex-a4114a
25. Micafungin Sodium [mart.]
26. Micafungin Sodium [who-dd]
27. Micafungin Sodium Salt [mi]
28. Mfcd08067752
29. S4287
30. Ccg-270668
31. Micafungin Sodium [orange Book]
32. Ac-30600
33. Bm164667
34. D02465
35. Q27149252
36. Pneumocandin A0, 1-((4r,5r)-4,5-dihydroxy-n2-(4-(5-(5-(pentyloxy)phenyl)-3-isoxazolyl)benzoyl)-l-ornithine)-4-((4s)-4-hydroxy-4-(4-hydroxy-3-(sulfooxy)phenyl)-l-threonine)-, Monosodium Salt
37. Pneumocandin A0, 1-(4r,5r)-4,5-ihydroxy-2-4-5-4-(pentyloxy)henyl]-3-soxazolyl]enzoyl]--rnithine]-4-(4s)-4-ydroxy-4-4-ydroxy-3-(sulfooxy)henyl]
| Molecular Weight | 1292.3 g/mol |
|---|---|
| Molecular Formula | C56H70N9NaO23S |
| Hydrogen Bond Donor Count | 15 |
| Hydrogen Bond Acceptor Count | 24 |
| Rotatable Bond Count | 18 |
| Exact Mass | 1291.42029497 g/mol |
| Monoisotopic Mass | 1291.42029497 g/mol |
| Topological Polar Surface Area | 521 Ų |
| Heavy Atom Count | 90 |
| Formal Charge | 0 |
| Complexity | 2580 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 15 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Mycamine |
| PubMed Health | Micafungin (Injection) |
| Drug Classes | Antifungal |
| Drug Label | Mycamine is a sterile, lyophilized product for intravenous (IV) infusion that contains micafungin sodium. Micafungin sodium is a semisynthetic lipopeptide (echinocandin) synthesized by a chemical modification of a fermentation product of Coleophoma e... |
| Active Ingredient | Micafungin sodium |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion) |
| Strength | 100mg/vial; 50mg; 50mg/vial |
| Market Status | Prescription |
| Company | Astellas; Fujisawa Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Mycamine |
| PubMed Health | Micafungin (Injection) |
| Drug Classes | Antifungal |
| Drug Label | Mycamine is a sterile, lyophilized product for intravenous (IV) infusion that contains micafungin sodium. Micafungin sodium is a semisynthetic lipopeptide (echinocandin) synthesized by a chemical modification of a fermentation product of Coleophoma e... |
| Active Ingredient | Micafungin sodium |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion) |
| Strength | 100mg/vial; 50mg; 50mg/vial |
| Market Status | Prescription |
| Company | Astellas; Fujisawa Hlthcare |
Mycamine is indicated for:
* Adults, adolescents 16 years of age and elderly:
- treatment of invasive candidiasis;
- treatment of oesophageal candidiasis in patients for whom intravenous therapy is appropriate;
- prophylaxis of Candida infection in patients undergoing allogeneic haematopoietic stem-cell transplantation or patients who are expected to have neutropenia (absolute neutrophil count < 500 cells/l) for 10 or more days.
* Children (including neonates) and adolescents < 16 years of age:
- treatment of invasive candidiasis.
- prophylaxis of Candida infection in patients undergoing allogeneic haematopoietic stem-cell transplantation or patients who are expected to have neutropenia (absolute neutrophil count < 500 cells/l) for 10 or more days.
The decision to use Mycamine should take into account a potential risk for the development of liver tumours. Mycamine should therefore only be used if other antifungals are not appropriate.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
J02AX05
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Medichem is a vertically integrated pharmaceutical company specializing in the development & manufacturing of APIs & FDFs.
Medichem is a vertically integrated pharmaceutical company specializing in the development & manufacturing of APIs & FDFs.

GDUFA
DMF Review : Reviewed
Rev. Date : 2016-09-20
Pay. Date : 2016-06-02
DMF Number : 30558
Submission : 2016-06-06
Status : Active
Type : II

NDC Package Code : 16436-0114
Start Marketing Date : 2014-07-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (75kg/75kg)
Marketing Category : BULK INGREDIENT
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-09-01
Pay. Date : 2020-08-07
DMF Number : 34888
Submission : 2020-07-31
Status : Active
Type : II

Registration Number : 301MF10054
Registrant's Address : No. 1191, Sec. 1, Chung Shan Rd. , Tachia, Taichung, Taiwan, R. O. C.
Initial Date of Registration : 2019-08-28
Latest Date of Registration : --

NDC Package Code : 63126-908
Start Marketing Date : 2020-09-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2018-10-29
Pay. Date : 2018-05-14
DMF Number : 32812
Submission : 2018-06-17
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-01-23
Pay. Date : 2013-09-13
DMF Number : 27435
Submission : 2013-09-25
Status : Active
Type : II

NDC Package Code : 58623-0113
Start Marketing Date : 2018-04-20
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12856
Submission : 1998-02-11
Status : Active
Type : II

Registrant Name : Astellas Pharma Korea Co., Ltd.
Registration Date : 2006-05-11
Registration Number : Number 118-1-ND
Manufacturer Name : Astellas Pharma Inc. Toyama Technology Center
Manufacturer Address : 2-178, Kojin-machi, Toyama-city, Toyama 930-0809, Japan (3-11, Nihenbashi-honcho 2chome, Chuo-ku, Tokyo 103-8411, Japan)

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?