
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
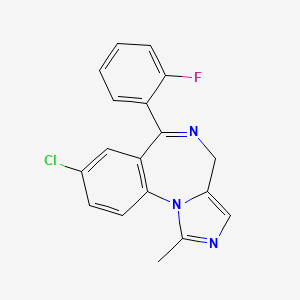

1. Dormicum
2. Hydrochloride, Midazolam
3. Maleate, Midazolam
4. Midazolam Hydrochloride
5. Midazolam Maleate
6. Ro 21 3981
7. Ro 21-3981
8. Ro 213981
9. Versed
1. Dormicum
2. 59467-70-8
3. Versed
4. 8-chloro-6-(2-fluorophenyl)-1-methyl-4h-imidazo[1,5-a][1,4]benzodiazepine
5. Midazolamum
6. Buccolam
7. Midazolamum [inn-latin]
8. Midazolam Base
9. Mezolam
10. Ro 21-3981
11. Nayzilam
12. Usl261
13. Dea No. 2884
14. Midazolam Civ
15. Chembl655
16. R60l0sm5bc
17. Chebi:6931
18. Usl-261
19. Hypnovel
20. 4h-imidazo(1,5-a)(1,4)benzodiazepine, 8-chloro-6-(2-fluorophenyl)-1-methyl-
21. 4h-imidazo[1,5-a][1,4]benzodiazepine, 8-chloro-6-(2-fluorophenyl)-1-methyl-
22. 8-chloro-6-(o-fluorophenyl)-1-methyl-4h-imidazo[1,5-a][1,4]-benzodiazepine
23. Dormicum (tn)
24. 12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triazatricyclo[8.4.0.0^{2,6}]tetradeca-1(10),3,5,8,11,13-hexaene
25. Ncgc00168254-01
26. Einecs 261-774-5
27. Unii-r60l0sm5bc
28. Brn 0625572
29. Medazolam
30. Midazolam [usp:inn:ban:jan]
31. 8-chloro-6-(2-fluorophenyl)-1-methyl-4h-imidazo(1,5-a)(1,4)benzodiazepine
32. Buccolam (tn)
33. Nayzilam (tn)
34. 08j
35. 8-chlor-6-(2-fluorphenyl)-1-methyl-4h-imidazo(1,5-a)(1,4)benzodiazepin
36. 8-chloro-6-(o-fluorophenyl)-1-methyl-4h-imidazo(1,5-a)(1,4)benzodiazepine
37. Versed (salt/mix)
38. Dormonid (salt/mix)
39. Hypnovel (salt/mix)
40. Flormidal (salt/mix)
41. Midazolam [inn]
42. Midazolam [jan]
43. Midazolam [mi]
44. Midazolam [vandf]
45. Midazolam [mart.]
46. Midazolam [who-dd]
47. Midazolam (jan/usp/inn)
48. Schembl35061
49. Bidd:gt0647
50. Midazolam Hydrochloride, Solid
51. Gtpl3342
52. Midazolam [orange Book]
53. Midazolam Civ [usp-rs]
54. Dtxsid5023320
55. Midazolam [ep Monograph]
56. Bdbm21363
57. Hsdb 6751
58. Midazolam [usp Monograph]
59. Midazolam 0.1 Mg/ml In Methanol
60. Midazolam 1.0 Mg/ml In Methanol
61. Usl261 (nasal Spray Formulation)
62. Bcp21296
63. Hy-b0676
64. 59467-70-8 (free)
65. Zinc95626706
66. Akos015842580
67. Db00683
68. 4h-imidazo[1,5-a][1,4]benzodiazepine, 8-chloro-6-(2-fluoro-phenyl)-1-methyl-, (z)-2-butenedioate
69. Db-015270
70. C07524
71. D00550
72. 467m640
73. A832332
74. Q423071
75. Midazolam Hydrochloride, United States Pharmacopeia (usp) Reference Standard
76. 4h-imidazo(1,5-a)(1,4)benzodiazepine, 8-chloro-6-(2-fluorophenyl)-1-methyl
77. Midazolam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
78. 12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triazatricyclo[8.4.0.0^{2,6}]tetradeca-1(14),3,5,8,10,12-hexaene
| Molecular Weight | 325.8 g/mol |
|---|---|
| Molecular Formula | C18H13ClFN3 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 325.0782033 g/mol |
| Monoisotopic Mass | 325.0782033 g/mol |
| Topological Polar Surface Area | 30.2 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 471 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adjuvants, Anesthesia; Anesthetics, Intravenous; Anti-Anxiety Agents; GABA Modulators; Hypnotics and Sedatives
National Library of Medicine's Medical Subject Headings. Midazolam. Online file (MeSH, 2014). Available from, as of August 28, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Midazolam is a familiar agent commonly used in the emergency department to provide sedation prior to procedures such as laceration repair and reduction of dislocations. Midazolam is also effective in the treatment of generalized seizures, status epilepticus, and behavioral emergencies, particularly when intravenous access is not available. Midazolam is often employed as an induction agent for rapid sequence endotracheal intubation...
PMID:9258787 Nordt SP and Clark RF. J Emerg Med. 15(3):357-65. (1997).
EXP THER: This study evaluated the anticonvulsant effectiveness of midazolam to stop seizures elicited by the nerve agent soman when midazolam was administered by different routes (intramuscular, intranasal or sublingual) at one of two different times after the onset of seizure activity. Guinea pigs previously prepared with cortical electrodes to record brain electroencephalographic activity were pre-treated with pyridostigmine (0.026 mg/kg, intramuscularly) 30 min. before challenge with a seizure-inducing dose of the nerve agent soman (56 microg/kg, subcutaneously), and 1 min. later, they were administered 2.0 mg/kg atropine sulfate admixed with 25.0 mg/kg 2-PAM Cl (intramuscularly). Groups of animals were administered differing doses of midazolam by the intramuscular, intranasal or sublingual route at either the onset of seizure activity or 40 min. after the onset of seizure activity that was detected in the electroencephalographic record. When given immediately after seizure onset, the anticonvulsant ED50 of intramuscular midazolam was significantly lower than that of intranasal midazolam, which in turn was significantly lower than sublingual midazolam at that time. At the 40-min. treatment delay, the anticonvulsant ED50s of intramuscular or intranasal midazolam did not differ and both were significantly lower than the sublingual route. Higher doses of midazolam were required to stop seizures at the 40-min. treatment delay time compared to immediate treatment. The speed of seizure control for intramuscular or intranasal midazolam was the same while sublingual midazolam acted significantly slower. Midazolam was effective in treating soman-induced seizures when given by all three routes, but with differences in potency and speed of action.
PMID:19053994 McDonough JH et al; Basic Clin Pharmacol Toxicol. 104(1):27-34. (2009).
For preoperative sedation and anxiolysis, and anterograde amnesia, midazolam is used IM or IV in adult or pediatric patients; the drug also is used orally in pediatric patients. When administered preoperatively, the drug relieves anxiety and provides sedation, light anesthesia, and anterograde amnesia of perioperative events.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2652
For more Therapeutic Uses (Complete) data for Midazolam (11 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: Adult and Pediatric: Intravenous midazolam has been associated with respiratory depression and respiratory arrest, especially when used for sedation in noncritical care settings. In some cases, where this was not recognized promptly and treated effectively, death or hypoxic encephalopathy has resulted. Intravenous midazolam should be used only in hospital or ambulatory care settings, including physicians' and dental offices, that provide for continuous monitoring of respiratory and cardiac function, i.e., pulse oximetry. Immediate availability of resuscitative drugs and age- and size-appropriate equipment for bag/valve/mask ventilation and intubation, and personnel trained in their use and skilled in airway management should be assured. For deeply sedated pediatric patients, a dedicated individual, other than the practitioner performing the procedure, should monitor the patient throughout the procedure. The initial intravenous dose for sedation in adult patients may be as little as 1 mg, but should not exceed 2.5 mg in a normal healthy adult. Lower doses are necessary for older (over 60 years) or debilitated patients and in patients receiving concomitant narcotics or other central nervous system (CNS) depressants. The initial dose and all subsequent doses should always be titrated slowly; administer over at least 2 minutes and allow an additional 2 or more minutes to fully evaluate the sedative effect. The use of the 1 mg/mL formulation or dilution of the 1 mg/mL or 5 mg/mL formulation is recommended to facilitate slower injection. Doses of sedative medications in pediatric patients must be calculated on a mg/kg basis, and initial doses and all subsequent doses should always be titrated slowly. The initial pediatric dose of midazolam for sedation/anxiolysis/amnesia is age, procedure, and route dependent. Neonates: Midazolam should not be administered by rapid injection in the neonatal population. Severe hypotension and seizures have been reported following rapid IV administration, particularly with concomitant use of fentanyl.
NIH; DailyMed. Current Medication Information for Midazolam (Midazolam Hydrochloride) Injection (Revised: July 2012). Available from, as of November 17, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=737361a0-8db1-4d3c-ba5e-44df3f49fa22
Serious cardiorespiratory adverse events have occurred after administration of midazolam. These have included respiratory depression, airway obstruction, oxygen desaturation, apnea, respiratory arrest and/or cardiac arrest, sometimes resulting in death or permanent neurologic injury. There have also been rare reports of hypotensive episodes requiring treatment during or after diagnostic or surgical manipulations particularly in adult or pediatric patients with hemodynamic instability. Hypotension occurred more frequently in the sedation studies in patients premedicated with a narcotic.
NIH; DailyMed. Current Medication Information for Midazolam (Midazolam Hydrochloride) Injection (Revised: July 2012). Available from, as of November 17, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=737361a0-8db1-4d3c-ba5e-44df3f49fa22
Serious respiratory adverse events have occurred after administration of oral midazolam hydrochloride syrup, most often when midazolam hydrochloride syrup was used in combination with other central nervous system depressants. These adverse events have included respiratory depression, airway obstruction, oxygen desaturation, apnea, and rarely, respiratory and/or cardiac arrest. When oral midazolam is administered as the sole agent at recommended doses respiratory depression, airway obstruction, oxygen desaturation, and apnea occur infrequently
NIH; DailyMed. Current Medication Information for Midazolam (Midazolam Hydrochloride) Injection (Revised: July 2012). Available from, as of November 17, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9864801b-6c92-45aa-9c0c-b4b49666c31d
... The immediate availability of specific reversal agents (flumazenil) is highly recommended. Vital signs should continue to be monitored during the recovery period. Because intravenous midazolam depresses respiration.. and because opioid agonists and other sedatives can add to this depression, midazolam should be administered as an induction agent only by a person trained in general anesthesia and should be used for sedation/anxiolysis/amnesia only in the presence of personnel skilled in early detection of hypoventilation, maintaining a patent airway and supporting ventilation.
NIH; DailyMed. Current Medication Information for Midazolam (Midazolam Hydrochloride) Injection (Revised: July 2012). Available from, as of November 17, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=737361a0-8db1-4d3c-ba5e-44df3f49fa22
For more Drug Warnings (Complete) data for Midazolam (39 total), please visit the HSDB record page.
**Intravenous** Indicated for promoting preoperative sedation, anxiolysis, anesthesia induction, or amnesia. **Intramuscular** Indicated for the treatment of status epilepticus in adults. **Nasal** Indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patients usual seizure pattern in patients with epilepsy 12 years of age and older.
FDA Label
Treatment of prolonged, acute, convulsive seizures in infants, toddlers, children and adolescents (from three months to less than 18 years).
Buccolam must only be used by parents / carers where the patient has been diagnosed to have epilepsy.
For infants between three and six months of age, treatment should be in a hospital setting where monitoring is possible and resuscitation equipment is available.
**General effects** Midazolam is a short-acting benzodiazepine central nervous system (CNS) depressant. Pharmacodynamic properties of midazolam and its metabolites, which are similar to those of other benzodiazepine drugs, include sedative, anxiolytic, amnestic, muscle relaxant, as well as hypnotic activities. Benzodiazepines enhance the inhibitory action of the amino acid neurotransmitter gamma-aminobutyric acid (GABA). Receptors for GABA are targeted by many important drugs that affect GABA function and are commonly used in the treatment of anxiety disorder, epilepsy, insomnia, spasticity, and aggressive behavior. **Sedation and memory** The onset of sedation after intramuscular administration in adults is 15 minutes, with maximal sedation occurring 30-60 minutes after injection. In one study of adults, when tested the following day, 73% of the patients who were administered midazolam intramuscularly had no recollection of memory cards shown 30 minutes following drug administration; 40% had no recollection of the memory cards shown 60 minutes after drug administration. Onset time of sedative effects in pediatric patients begins within 5 minutes and peaks at 15-30 minutes depending upon the dose administered. In the pediatric population, up to 85% had no memory of pictures shown after receiving intramuscular midazolam compared to 5% of the placebo control group. Sedation in both adult and pediatric patients is reached within 3 to 5 minutes post intravenous (IV) injection. The time of onset is affected by the dose administered and the simultaneous administration of narcotic pre-medication. Seventy-one (71%) percent of the adult patients in clinical endoscopy studies had no memory of insertion of the endoscope; 82% of the patients had no memory of withdrawal of the endoscope. **Anesthesia induction** When midazolam is administered intravenously (IV) for anesthetic induction, induction of anesthesia occurs in about 1.5 minutes when narcotic pre-medication has been given and in 2 to 2.5 minutes without narcotic pre-medication/ other sedative pre-medication. Impairment in a memory test was observed in 90% of the patients.
Anesthetics, Intravenous
Ultrashort-acting anesthetics that are used for induction. Loss of consciousness is rapid and induction is pleasant, but there is no muscle relaxation and reflexes frequently are not reduced adequately. Repeated administration results in accumulation and prolongs the recovery time. Since these agents have little if any analgesic activity, they are seldom used alone except in brief minor procedures. (From AMA Drug Evaluations Annual, 1994, p174) (See all compounds classified as Anesthetics, Intravenous.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
N05CD08
N05CD08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CD - Benzodiazepine derivatives
N05CD08 - Midazolam
Absorption
**Oral Absorption**: Rapidly absorbed after oral administration. The absolute bioavailability, if given intramuscularly (IM), is greater than 90%. Due to first pass metabolism, only 40-50% of the administered oral dose reaches the circulation. The absolute bioavailability of the midazolam syrup in pediatric patients is about 36%. **Intramuscular Absorption**: The mean peak concentration (Cmax) and time to peak (Tmax) following the IM dose was 90 ng/mL (20% CV) and 0.5 hour (50% CV). **Rectal administration**: After rectal administration midazolam is absorbed rapidly. Maximum plasma concentration is reached within 30 minutes. The absolute bioavailability is approximately 50%. **Intranasal Administration**: Midazolam is absorbed rapidly after intranasal administration. Mean peak plasma concentrations are reached within 10.2 to 12.6 minutes. The bioavailability is between 55 and 57%.
Route of Elimination
The _-hydroxymidazolam_ glucuronide conjugate of midazolam is excreted in urine. No significant amount of parent drug or metabolites is found in urine before beta-glucuronidase and sulfatase deconjugation, suggesting that the urinary metabolites are excreted mainly as conjugates. The amount of midazolam excreted unchanged in the urine when given intravenously is less than 0.5%. 45% to 57% of the dose was excreted in the urine as 1-hydroxymethyl midazolam conjugate. Plasma clearance of midazolam is higher in patients that remain in supine position, because of a 40-60 percent increase in hepatic blood flow during supination. Pregnancy may also increase the metabolism of midazolam.
Volume of Distribution
Female gender, old age, and obesity may increase the volume of distribution. Midazolam may also cross the placenta and has been detected in human milk and cerebrospinal fluid. **Intravenous administration** 1.24 to 2.02 L/kg [pediatric patients (6 months to <16 years) receiving 0.15 mg/kg IV midazolam] 1 to 3.1 L/kg [midazolam intravenously administered, healthy adults]. **Intramuscular administration** The mean apparent volume of distribution of midazolam after a single IM dose of 10 mg midazolam in healthy adults was 2117 (845.1) mL/kg.
Clearance
**Intramuscular**: apparent total body clearance, 367.3 (73.5) mL/hr/kg. **Intravenous**: total clearance (Cl), 0.25 to 0.54 L/hr/kg
Following iv administration of midazolam hydrochloride in animals, the drug is widely distributed, with highest concentrations occurring in liver, kidneys, lungs, fat, and heart. The drug crosses the blood-brain barrier and distributes into cerebrospinal fluid in humans and animals. In animals, equilibration of midazolam between plasma and cerebrospinal fluid occurs within a few minutes following iv administration, and cerebrospinal fluid:plasma ratios of the drug are highly correlated with unbound midazolam once equilibrium is reached. Distribution of the drug into human lumbar cerebrospinal fluid may be slow and erratic. Distribution may be altered in geriatric patients.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2659-60
Absorption of midazolam hydrochloride from IM injection sites is rapid and nearly complete (mean absolute bioavailability is greater than 90%). Im bioavailability of the lactate appears to be similar to or slightly less than that of the hydrochloride; however, any such difference does not appear to be clinically important. Pharmacologic effects of midazolam usually are apparent within 5-15 min but may not be maximal until 15-60 min following IM administration; the duration of action usually is about 2 hr (range: 1-6 hr). Peak plasma midazolam concentrations generally are attained within 45 minutes following IM administration. Following administration of a single 12.5 mg (of midazolam) dose of the hydrochloride in healthy adults, peak plasma midazolam concentrations of approximately 200 ng/mL (range: 88-269 ng/mL) are attained. Peak plasma concentrations of midazolam and 1-hydroxymethylmidazolam (an active metabolite) attained following IM injections are approximately 50% of those attained following iv injection of a dose.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1659
Midazolam hydrochloride is absorbed rapidly from the GI tract, with maximum plasma concentration usually occurring within 1-2 hours. Following oral administration, the drug undergoes substantial first-pass metabolism in the liver and intestine, with only about 40-50% (range: 28-72%) of an orally administered dose reaching systemic circulation unchanged.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2659
At physiologic pH, midazolam is highly lipophilic; however, the lipophilicity of the drug decreases with decreasing pH. Following iv administration in humans, midazolam is rapidly and apparently widely distributed. The apparent volume of distribution of the drug in healthy adults reportedly averages 0.8-2.5 L/kg (range: 0.6-6.6 L/kg). Volume of distribution of midazolam appears to be 1.5 to 2 times higher in adults with chronic renal failure and 2 to 3 times higher in adults with congestive heart failure compared with healthy adults.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2659
For more Absorption, Distribution and Excretion (Complete) data for Midazolam (9 total), please visit the HSDB record page.
Midazolam is primarily metabolized in the liver and gut by CYP3A4 to its pharmacologic active metabolite, _alpha-hydroxymidazolam_ (also known as 1-hydroxy-midazolam), and 4-hydroxymidazolam (which makes up 5% or less of the biotransformation products). This metabolite likely contributes to the pharmacological effects of midazolam. Midazolam also undergoes N-glucuronidation via UGT1A4 after the process of hepatic oxidation by cytochrome enzymes.
... Midazolam has a rapid onset of action following intravenous, intramuscular, oral, nasal, and rectal administration. Only 50% of an orally administered dose reaches the systemic circulation due to extensive first-pass metabolism. Midazolam is metabolized by the cytochrome P450 enzyme system to several metabolites including an active metabolite, alpha-hydroxymidazolam. Cytochrome P450 inhibitors such as cimetidine can profoundly reduce the metabolism of midazolam...
PMID:9258787 Nordt SP, Clark RF: J Emerg Med 15 (3): 357-65 (1997)
Midazolam is a short-acting benzodiazepine routinely used in intensive-care medicine. Conjugates of its main metabolite, alpha-hydroxymidazolam, have been shown to accumulate in renal failure but have not previously been related to the prolonged sedative effects commonly observed in critically ill patients. /This study reports on/ five patients with severe renal failure who had prolonged sedation after administration of midazolam. In all five patients, the comatose state was immediately reversed by the benzodiazepine-receptor antagonist flumazenil. Serum concentration monitoring showed high concentrations of conjugated alpha-hydroxymidazolam when concentrations of the unconjugated metabolite and the parent drug were below the therapeutic range. In-vitro binding studies showed that the affinity of binding to the cerebral benzodiazepine receptor of glucuronidated alpha-hydroxymidazolam was only about ten times weaker (affinity constant 16 nmol/L) than that of midazolam (1.4 nmol/L) or unconjugated alpha-hydroxymidazolam (2.2 nmol/L). Conjugated metabolites of midazolam have substantial pharmacological activity. Physicians should be aware that these metabolites can accumulate in patients with renal failure.
PMID:7603229 Bauer TM et al; Lancet 346: 145-147 (1995)
The kinetics and dynamics of midazolam were investigated in 20 female patients undergoing lower abdominal surgery. The relation between the plasma concentrations of midazolam and pharmacokinetic end points was evaluated after an intravenous infusion regimen in 10 patients given an epidural anesthetic. The remaining 10 patients were anesthetized with a totally intravenous anesthetic technique with midazolam and alfentanil. The effect was assessed by means of a rating scale divided into degree of sedation and amnesia. A good correlation was found between plasma level of midazolam and pharmacodynamic response. The relation between the quantal response data and the plasma concentration was represented by an s-shaped concentration-effect curve. Despite similar kinetics of midazolam in the two groups, the postoperative drowsiness was more pronounced in the group receiving total intravenous anesthesia. The concomitant administration of alfentanil shifted the concentration-effect curve regarding sedation to the left.
PMID:3126014 Persson MP et al; Clin Pharmacol Ther 43: 324-31 (1988)
Midazolam is metabolized extensively in the liver and intestine by cytochrome P-450 CYP3A4. The drug rapidly undergoes hydroxylation via hepatic microsomal enzymes to form 1-hyroxymethylmidazolam (alpha-hydroxymidazolam), the principal metabolite, and 4-hydroxymidazolam; a small portion of 1-hydroxymethylmidzaolam is further hydroxylated to 1-hydroxymethyl-4-hydroxymidazolam (alpha,4-dihydroxymidazolam). These metabolites undergo rapid conjugation with glucuronic acid in the liver. Although the elimination half-life of the principal metabolite, 1-hydroxymethylmidazolam, is not clearly established, it is estimated to be about 60-80 min. The 1-hydroxymethyl and 4-hydroxy metabolites are reportedly pharmacologically active, but their potencies at equivalent molar concentrations appear to be substantially less than that of midazolam. The 1-hydroxymethyl-4-hydroxy metabolite appears to have little, if any, pharmacologic activity.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2660
For more Metabolism/Metabolites (Complete) data for Midazolam (6 total), please visit the HSDB record page.
Midazolam has known human metabolites that include 1'-Hydroxymidazolam, 4-Hydroxymidazolam, and Midazolam N-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
**Intravenous**: healthy adults = 1.8 to 6.4 hours (mean of 3 hours). **Intramuscular**: Following IM administration of 10 mg midazolam, mean (SD) elimination half-life was 4.2 (1.87) hours.
Midazolam is extensively protein bound (more than 95%). With an intravenous dose of 0.075 mg/kg, the half-life is 68 min, the apparent volume of distribution is 0.23 L/kg, and the clearance is 13 mL/kg/min. The half-life is prolonged in patients with cirrhosis.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 76
...Most elimination half-life ranged from 2.9-4.5 hours in pediatric patients (6 months to less than 16 years of age) receiving IV midazolam 150 ug/kg. In seriously ill neonates, the terminal elimination half-life is substantially prolonged (ie. 6.5-12 hours).
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2660
Following a single IV dose in health adults, the half-life of midazolam in the initial distribution phase (t 1/2 alpha) averages 6-20 minutes, and the half-life in the terminal elimination phase (t 1/2 beta) averages 1-4 hours (range: 1-12.3 hours). Limited data suggest that the half-life of midazolam may be prolonged in obese patients (presumably secondary to an increased volume of distribution), geriatric individuals, and patients with impaired hepatic function or with congestive heart failure. The half-life of midazolam is also repeatedly prolonged in patients receiving the drug for induction of anesthesia associated with major surgical procedures...
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2660
The actions of benzodiazepines such as midazolam are mediated through the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), which is one of the major inhibitory neurotransmitters in the central nervous system. Benzodiazepines increase the activity of GABA, thereby producing a sedating effect, relaxing skeletal muscles, and inducing sleep, anesthesia, and amnesia. Benzodiazepines bind to the benzodiazepine site on GABA-A receptors, which potentiates the effects of GABA by increasing the frequency of chloride channel opening. These receptors have been identified in different body tissues including the heart and skeletal muscle, although mainly appear to be present in the central nervous system.
Midazolam has neurotoxic properties when administered neuraxially in vivo. Furthermore, midazolam induces neurodegeneration in neonatal animal models in combination with other general anesthetics. Therefore, this study focuses on the mechanism of neurotoxicity by midazolam in neuronal and nonneuronal cells. The study aims to evaluate the apoptotic pathway and to investigate the protective effects of the benzodiazepine antagonist flumazenil and the caspase inhibitor N-(2-quinolyl)valyl-aspartyl-(2,6-difluorophenoxy)-methylketone. The apoptosis-inducing effect of preservative-free midazolam on human lymphoma and neuroblastoma cell lines was evaluated using flow cytometric analysis of early apoptotic stages (annexin V/7AAD) and caspase 3 activation. B-cell lymphoma (Bcl2) protein overexpressing and caspase 9-deficient lymphoma cells were used to determine the role of the mitochondrial (intrinsic) pathway. Caspase 8-deficient and Fas-associated protein with death domain (FADD)-deficient cells were used to evaluate the death receptor (extrinsic) pathway. The protective effects of flumazenil and the caspase inhibitor N-(2-quinolyl)valyl-aspartyl-(2,6-difluorophenoxy)-methylketone were investigated in neuroblastoma cells and primary rat neurons using metabolic activity assays (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) and immunofluorescence microscopy. Midazolam induced apoptosis in all investigated cell types in a concentration-dependent manner, indicated by flow cytometry. Bcl2-overexpression and caspase 9 deficiency protected against toxicity, whereas caspase 8 or FADD deficiency had no effect. Pancaspase inhibition had a strong protective effect, whereas flumazenil did not inhibit midazolam-induced apoptosis. Midazolam induces apoptosis via activation of the mitochondrial pathway in a concentration-dependent manner. The mechanism of midazolam toxicity switches from caspase-dependent apoptosis to necrosis with increasing concentrations. The induction of apoptosis and necrosis by midazolam is presumably unrelated to GABAA receptor pathway signaling.
PMID:21701267 Stevens MF et al; Reg Anesth Pain Med 36 (4): 343-9 (2011)
A consummatory conflict procedure that involves an abrupt reduction in magnitude of an expected reward (negative contrast) has been shown to be particularly sensitive to the effects of anxiolytic agents. Midazolam released suppressed consummatory performance in a dose-dependent manner. This effect was not due to a general appetite stimulation effect of the drug. The effects of three 5-HT antagonists on negative contrast were examined to evaluate the role serotonin may play in the anxiolytic action of benzodiazepine. Methysergide was found to be ineffective, cinanserin tended to reduce contrast at two intermediate doses, and cyproheptadine eliminated the contrast effect in a similar fashion as midazolam. The effectiveness of cyproheptadine may not be attributed to its anticholinergic or antihistaminergic actions since scopolamine and pyrilamine did not produce similar efects. The results are discussed in terms of the role serotonin may play in the anti-conflict action of benzodiaepine, as well as possible interactional effects of gamma-aminobutyric acid.
PMID:3012590 Becker HC; Pharmacol Biochem Behav 24 (4): 1057-64 (1986)
The exact sites and mode of action of the benzodiazepines have not been fully elucidated, but the effects of the drugs appear to be mediated through the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). The drugs appear to act at the limbic, thalamic, and hypothalamic levels of the CNS, producing anxiolytic, sedative, hypnotic, skeletal muscle relaxant, and anticonvulsant effects. Benzodiazepines are capable of producing all levels of CNS depression-from mild sedation to hypnosis to coma. /Benzodiazepines/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2642
Benzodiazepines appear to produce skeletal muscle relaxation predominantly by inhibiting spinal polysynaptic afferent pathways, but the drugs may also inhibit monosynaptic afferent pathways. The drugs may inhibit monosynaptic and polysynaptic reflexes by acting as inhibitory neuronal transmitters or by blocking excitatory synaptic transmission. The drugs may also directly depress motor nerve and muscle function. /Benzodiazepines/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2642
For more Mechanism of Action (Complete) data for Midazolam (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
77
PharmaCompass offers a list of Midazolam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Midazolam manufacturer or Midazolam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Midazolam manufacturer or Midazolam supplier.
PharmaCompass also assists you with knowing the Midazolam API Price utilized in the formulation of products. Midazolam API Price is not always fixed or binding as the Midazolam Price is obtained through a variety of data sources. The Midazolam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Midazolam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Midazolam, including repackagers and relabelers. The FDA regulates Midazolam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Midazolam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Midazolam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Midazolam supplier is an individual or a company that provides Midazolam active pharmaceutical ingredient (API) or Midazolam finished formulations upon request. The Midazolam suppliers may include Midazolam API manufacturers, exporters, distributors and traders.
click here to find a list of Midazolam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Midazolam DMF (Drug Master File) is a document detailing the whole manufacturing process of Midazolam active pharmaceutical ingredient (API) in detail. Different forms of Midazolam DMFs exist exist since differing nations have different regulations, such as Midazolam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Midazolam DMF submitted to regulatory agencies in the US is known as a USDMF. Midazolam USDMF includes data on Midazolam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Midazolam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Midazolam suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Midazolam Drug Master File in Japan (Midazolam JDMF) empowers Midazolam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Midazolam JDMF during the approval evaluation for pharmaceutical products. At the time of Midazolam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Midazolam suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Midazolam Drug Master File in Korea (Midazolam KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Midazolam. The MFDS reviews the Midazolam KDMF as part of the drug registration process and uses the information provided in the Midazolam KDMF to evaluate the safety and efficacy of the drug.
After submitting a Midazolam KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Midazolam API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Midazolam suppliers with KDMF on PharmaCompass.
A Midazolam CEP of the European Pharmacopoeia monograph is often referred to as a Midazolam Certificate of Suitability (COS). The purpose of a Midazolam CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Midazolam EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Midazolam to their clients by showing that a Midazolam CEP has been issued for it. The manufacturer submits a Midazolam CEP (COS) as part of the market authorization procedure, and it takes on the role of a Midazolam CEP holder for the record. Additionally, the data presented in the Midazolam CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Midazolam DMF.
A Midazolam CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Midazolam CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Midazolam suppliers with CEP (COS) on PharmaCompass.
A Midazolam written confirmation (Midazolam WC) is an official document issued by a regulatory agency to a Midazolam manufacturer, verifying that the manufacturing facility of a Midazolam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Midazolam APIs or Midazolam finished pharmaceutical products to another nation, regulatory agencies frequently require a Midazolam WC (written confirmation) as part of the regulatory process.
click here to find a list of Midazolam suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Midazolam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Midazolam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Midazolam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Midazolam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Midazolam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Midazolam suppliers with NDC on PharmaCompass.
Midazolam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Midazolam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Midazolam GMP manufacturer or Midazolam GMP API supplier for your needs.
A Midazolam CoA (Certificate of Analysis) is a formal document that attests to Midazolam's compliance with Midazolam specifications and serves as a tool for batch-level quality control.
Midazolam CoA mostly includes findings from lab analyses of a specific batch. For each Midazolam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Midazolam may be tested according to a variety of international standards, such as European Pharmacopoeia (Midazolam EP), Midazolam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Midazolam USP).

