
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
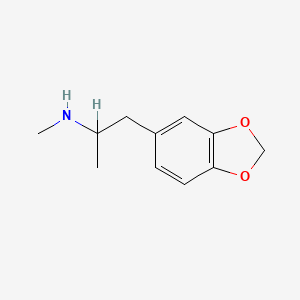

1. Ecstasy (drug)
2. Hydrochloride, N-methyl-3,4-methylenedioxyamphetamine
3. Mdma
4. Methylenedioxymethamphetamine
5. N Methyl 3,4 Methylenedioxyamphetamine
6. N Methyl 3,4 Methylenedioxyamphetamine Hydrochloride
7. N-methyl-3,4-methylenedioxyamphetamine
8. N-methyl-3,4-methylenedioxyamphetamine Hydrochloride
1. Mdma
2. Ecstasy
3. 42542-10-9
4. Midomafetamine
5. Methylenedioxymethamphetamine
6. N-methyl-3,4-methylenedioxyamphetamine
7. Dl-(3,4-methylenedioxy)methamphetamine
8. (rs)-3,4-(methylenedioxy)methamphetamine
9. Mdma (unspecified)
10. N,alpha-dimethyl-1,3-benzodioxole-5-ethanamine
11. 1-(1,3-benzodioxol-5-yl)-n-methylpropan-2-amine
12. Hsdb 6929
13. Chebi:1391
14. 1,3-benzodioxole-5-ethanamine, N,alpha-dimethyl-
15. Dea No. 7405
16. 1-(1,3-benzodioxol-5-yl)-n-methyl-2-propanamine
17. (+-)-(3,4-methylenedioxy)methamphetamine
18. Midomafetamine [usan]
19. Ke1sen21rm
20. (+-)-n-methyl-3,4-(methylenedioxy)amphetamine
21. Methylenedioxymetamphetamine
22. Methylenedioxymethamfetamine
23. Phenethylamine, N,alpha-dimethyl-3,4-methylenedioxy-
24. Chembl43048
25. 3,4-methylenedioxy-n,alpha-dimethyl-beta-phenylethylamine
26. (+-)-n-methyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane
27. 3,4-methylenedioxymetamphetamine
28. Xtc
29. (+/-)-(3,4-methylenedioxy)methamphetamine
30. 1,3-benzodioxole-5-ethanamine, N,.alpha.-dimethyl-
31. Xtc [street Name]
32. Mandy [street Name]
33. Ncgc00168266-02
34. Unii-ke1sen21rm
35. Ccris 9277
36. Epitope Id:178091
37. Mdma [mi]
38. Midomafetamine (usan/inn)
39. Midomafetamine [inn]
40. (+/-)-3,4-methylenedioxymethamphetamine
41. Schembl44210
42. Methylenedioxy Methamphetamine
43. Divk1c_000962
44. 3,4-mdma
45. Gtpl4574
46. Kbio1_000962
47. Dtxsid90860791
48. Ninds_000962
49. 3,4-methylenedioxymethylamphetamine
50. Bdbm50010588
51. Pdsp1_001522
52. Pdsp2_001506
53. Rac-3,4-methylenedioxymethamphetamine
54. Akos006283463
55. Db01454
56. Idi1_000962
57. Ncgc00168266-01
58. Methylenedioxymethamfetamine [mart.]
59. D,l-3,4-methylenedioxymethamphetamine
60. Methylenedioxymethamphetamine [who-dd]
61. 3,4-methylenedioxy-n-methylamphetamine (mdma)
62. C07577
63. D11172
64. Q69488
65. (+/-)-n-methyl-3,4-(methylenedioxy)amphetamine
66. N-methyl-3,4-methylenedioxyamphetamine [hsdb]
67. [1-(2h-1,3-benzodioxol-5-yl)propan-2-yl](methyl)amine
68. Phenethylamine, N,.alpha.-dimethyl-3,4-methylenedioxy-
69. (rs)-1-(benzo(d)(1,3)dioxol-5-yl)-n-methylpropan-2-amine
70. (+/-)-n,.alpha.-dimethyl-3,4-(methylenedioxy)phenethylamine
71. (+/-)-n-methyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane
72. Rac-mdma (rac-3,4-methylenedioxymethamphetamine) 1.0 Mg/ml In Methanol
73. (+/-)-mdma Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
74. 54946-52-0
| Molecular Weight | 193.24 g/mol |
|---|---|
| Molecular Formula | C11H15NO2 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 193.110278721 g/mol |
| Monoisotopic Mass | 193.110278721 g/mol |
| Topological Polar Surface Area | 30.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 186 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Hallucinogens Serotonin Agents; Adrenergic Uptake Inhibitors
National Library of Medicine's Medical Subject Headings. N-Methyl-3,4-methylenedioxyamphetamine. Online file (MeSH, 2017). Available from, as of October 6, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. MDMA is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of October 6, 2017: https://clinicaltrials.gov/
/EXPL THER/ The first study of 3,4-methylenedioxymethamphetamine (MDMA)-assisted therapy for the treatment of social anxiety in autistic adults commenced in the spring of 2014. The search for psychotherapeutic options for autistic individuals is imperative considering the lack of effective conventional treatments for mental health diagnoses that are common in this population. Serious Adverse Events (SAEs) involving the administration of MDMA in clinical trials have been rare and non-life threatening. To date, MDMA has been administered to over 1133 individuals for research purposes without the occurrence of unexpected drug-related SAEs that require expedited reporting per FDA regulations. Now that safety parameters for limited use of MDMA in clinical settings have been established, a case can be made to further develop MDMA-assisted therapeutic interventions that could support autistic adults in increasing social adaptability among the typically developing population. As in the case with classic hallucinogens and other psychedelic drugs, MDMA catalyzes shifts toward openness and introspection that do not require ongoing administration to achieve lasting benefits. This infrequent dosing mitigates adverse event frequency and improves the risk/benefit ratio of MDMA, which may provide a significant advantage over medications that require daily dosing. Consequently, clinicians could employ new treatment models for social anxiety or similar types of distress administering MDMA on one to several occasions within the context of a supportive and integrative psychotherapy protocol.
PMID:25818246 Danforth AL et al; Prog Neuropsychopharmacol Biol Psychiatry 64: 237-49 (2016)
/EXPL THER/ l-3,4-dihydroxyphenylalanine (l-DOPA) is the most effective treatment for Parkinson's disease, but long-term l-DOPA administration is marred by the emergence of motor complications, namely, dyskinesia and a shortening of antiparkinsonian benefit (wearing-OFF). 3,4-methylenedioxymethamphetamine (MDMA) is unique in that it exerts antidyskinetic effects and may enhance antiparkinsonian actions of l-DOPA. MDMA is composed of two enantiomers with different pharmacological profiles; here, we describe a novel enantiospecific synthesis of the two enantiomers and expand on the previous characterization of their pharmacology. R-MDMA (rectus-MDMA) is relatively selective for 5-HT(2A) receptors, whereas S-MDMA (sinister-MDMA) inhibits both serotonin (SERT) and dopamine transporters (DAT; SERT/DAT ratio of 10 to 1). R- or S-MDMA (1, 3, and 10 mg/kg, s.c.) was administered in combination with l-DOPA (15 mg/kg, s.c.) to six female common marmosets (Callithrix jacchus) rendered parkinsonian by MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) administration. Motor disability, including parkinsonism and dyskinesia, and duration of antiparkinsonian benefit (ON-time) were evaluated. After the administration of R-MDMA (3 and 10 mg/kg), the severity of peak-dose dyskinesia was decreased (by 33 and 46%, respectively; p < 0.05); although total ON-time was unchanged (approximately 220 min), the duration of ON-time with disabling dyskinesia was decreased by 90 min when compared to l-DOPA alone (69% reduction; p < 0.05). S-MDMA (1 mg/kg) increased the total ON-time by 88 min compared to l-DOPA alone (34% increase; p < 0.05), though dyskinesia were exacerbated. These data suggest that racemic MDMA exerts simultaneous effects, reducing dyskinesia and extending ON-time, by 5-HT(2A) antagonism and SERT-selective mixed monoamine uptake inhibition, which arise from its R and S enantiomers, respectively.
PMID:21562283 Huot P et al; J Neurosci 31 (19): 7190-8 (2011)
For more Therapeutic Uses (Complete) data for 3,4-Methylenedioxymethamphetamine (7 total), please visit the HSDB record page.
The toxic dose is variable, with near fatal and fatal ingestions having been reported with blood levels between 0.11 mg/L to 2.1 mg/L. Survival has also been reported after MDMA blood levels of 4.3 mg/L drawn 13 hours after ingestion.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1075
Clinical trials are now testing the therapeutic potential of MDMA for post-traumatic stress disorder (PTSD) and anxiety associated with terminal cancer. MDMA is one of the four most widely used illicit drugs in the U.S.
MDMA acts as a releasing agent of serotonin, norepinephrine, and dopamine.
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
Hallucinogens
Drugs capable of inducing illusions, hallucinations, delusions, paranoid ideations, and other alterations of mood and thinking. Despite the name, the feature that distinguishes these agents from other classes of drugs is their capacity to induce states of altered perception, thought, and feeling that are not experienced otherwise. (See all compounds classified as Hallucinogens.)
Serotonin Agents
Drugs used for their effects on serotonergic systems. Among these are drugs that affect serotonin receptors, the life cycle of serotonin, and the survival of serotonergic neurons. (See all compounds classified as Serotonin Agents.)
Route of Elimination
renal
3,4-Methylenedioxymethamphetamine (MDMA), or ecstasy, is excreted as unchanged drug, 3,4-methylenedioxyamphetamine (MDA), and free and glucuronidated/sulfated 4-hydroxy-3-methoxymethamphetamine (HMMA), and 4-hydroxy-3-methoxyamphetamine (HMA) metabolites. The aim of this paper is to describe the pattern and timeframe of excretion of MDMA and its metabolites in urine. Placebo, 1.0 mg/kg, and 1.6 mg/kg oral MDMA doses were administered double-blind to healthy adult MDMA users on a monitored research unit. All urine was collected, aliquots were hydrolyzed, and analytes quantified by gas chromatography-mass spectrometry. Median C(max), T(max), ratios, first and last detection times, and detection rates were determined. Sixteen participants provided 916 urine specimens. After 1.6 mg/kg, median C(max) were 21,470 (MDMA), 2229 (MDA), 20,793 (HMMA), and 876 ng/mL (HMA) at median T(max) of 13.9, 23.0, 9.2 and 23.3 hr. In the first 24 hr, 30.2-34.3% total urinary excretion occurred. HMMA last detection exceeded MDMA's by more than 33 hr after both doses. Identification of HMMA as well as MDMA increased the ability to identify positive specimens but required hydrolysis. These MDMA, MDA, HMMA, and HMA pharmacokinetic data may be useful for interpreting workplace, drug treatment, criminal justice, and military urine drug tests. Measurement of urinary HMMA provides the longest detection of MDMA exposure yet is not included in routine monitoring procedures.
PMID:19874650 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3159864 Abraham TT et al; J Anal Toxicol 33 (8): 439-46 (2009)
Based on animal data, there is speculation that (+ or -)-3,4-methylenedioxymethamphetamine (MDMA) is neurotoxic to humans. Extrapolation of MDMA findings from animals to humans requires assessment of pharmacokinetics in various species, and low-dose administration data from rats are lacking. In this study, we examine MDMA pharmacokinetics in rats given low (2 mg/kg) and high (10 mg/kg) doses of racemic MDMA via intraperitoneal, subcutaneous, and oral routes. Repeated blood specimens were collected from venous catheters, and plasma was assayed for MDMA and its metabolites, 4-hydroxy-3-methoxymethamphetamine (HMMA) and 3,4-methylenedioxyamphetamine (MDA), by gas chromatography-mass spectrometry. After 2 mg/kg, maximum MDMA concentrations (C(max)) were approximately 200 ng/mL for intraperitoneal and subcutaneous routes, but less for the oral route. MDMA plasma half-lives were <1 hr for low-dose groups, whereas HMMA and MDA half-lives were >2 hr. After 10 mg/kg, MDMA areas under the curve (AUCs) were 21-fold (intraperitoneal), 10-fold (subcutaneous), and 36-fold (oral) greater than those at 2 mg/kg. In contrast, HMMA AUC values in high-dose groups were <3-fold above those at 2 mg/kg. Several new findings emerge from this report of low-dose MDMA pharmacokinetics in rats. First, 2 mg/kg MDMA in rats can produce MDMA C(max) values similar to those in humans, perhaps explaining why both species discriminate 1.5 mg/kg MDMA in laboratory paradigms. Second, our data provide additional support for nonlinear kinetics of MDMA in rats, and, analogous to humans, this phenomenon appears to involve impaired drug metabolism. Finally, given key similarities between MDMA pharmacokinetics in rats and humans, data from rats may be clinically relevant when appropriate dosing conditions are used.
PMID:19679675 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774984 Baumann MH et al; Drug Metab Dispos 37 (11): 2163-70 (2009)
3,4-Methylenedioxymethamphetamine (MDMA) is a widely abused illicit drug that can cause severe and even fatal adverse effects. However, interest remains for its possible clinical applications in posttraumatic stress disorder and anxiety treatment. Preclinical studies to determine MDMA's safety are needed. We evaluated MDMA's pharmacokinetics and metabolism in male rats receiving 2.5, 5, and 10 mg/kg s.c. MDMA, and the associated pharmacodynamic consequences. Blood was collected via jugular catheter at 0, 0.5, 1, 2, 4, 6, 8, 16, and 24 hours, with simultaneous serotonin (5-HT) behavioral syndrome and core temperature monitoring. Plasma specimens were analyzed for MDMA and the metabolites (+/-)-3,4-dihydroxymethamphetamine (HHMA), (+/-)-4-hydroxy-3-methoxymethamphetamine (HMMA), and (+/-)-3,4-methylenedioxyamphetamine (MDA) by liquid chromatography-tandem mass spectrometry. After 2.5 mg/kg MDMA, mean MDMA Cmax was 164 +/- 47.1 ng/mL, HHMA and HMMA were major metabolites, and <20% of MDMA was metabolized to MDA. After 5- and 10-mg/kg doses, MDMA areas under the curve (AUCs) were 3- and 10-fold greater than those after 2.5 mg/kg; HHMA and HMMA AUC values were relatively constant across doses; and MDA AUC values were greater than dose-proportional. Our data provide decisive in vivo evidence that MDMA and MDA display nonlinear accumulation via metabolic autoinhibition in the rat. Importantly, 5-HT syndrome severity correlated with MDMA concentrations (r = 0.8083; P < 0.0001) and core temperature correlated with MDA concentrations (r = 0.7595; P < 0.0001), suggesting that MDMA's behavioral and hyperthermic effects may involve distinct mechanisms. Given key similarities between MDMA pharmacokinetics in rats and humans, data from rats can be useful when provided at clinically relevant doses.
PMID:24141857 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3876787 Concheiro M et al; Drug Metab Dispos 42 (1): 119-25 (2014)
MDMA is rapidly absorbed into the human bloodstream, but once in the body, MDMA metabolites interfere with the body's ability to metabolize, or break down, the drug. As a result, additional doses of MDMA can produce unexpectedly high blood levels, which could worsen the cardiovascular and other toxic effects of this drug.
DHHS/NIH; NIDA Research Report Series on MDMA (Ecstasy) Abuse p.14 (2006). Available from, as of October 6, 2017: https://www.nida.nih.gov/PDF/RRmdma.pdf
For more Absorption, Distribution and Excretion (Complete) data for 3,4-Methylenedioxymethamphetamine (9 total), please visit the HSDB record page.
Midomafetamine, or MDMA, is reported to undergo extensive CYP-mediated hepatic metabolism, with CYP2D6 playing a major role in humans. Other CYP enzymes contributing to MDMA metabolism are CYP3A4 and COMT. MDMA is metabolized via two primary metabolic pathways. It may undergo O-demethylenation followed by catechol-O-methyltransferase (COMT)-catalyzed methylation and/or glucuronide/sulfate conjugation. In contrast, it may also undergo N-dealkylation, deamination, and oxidation to the corresponding benzoic acid derivatives conjugated with glycine. Due to autoinhibition of CYP2D6 and CYP2D8, MDMA displays a complex, nonlinear pharmacokinetics profile, with the zeroth order kinetics occurring at higher doses. It is thought that this can result in sustained and higher concentrations of MDMA if the user takes consecutive doses of the drug.
MDMA (3,4-methylenedioxymethamphetamine) metabolism is a major cause of MDMA-mediated hepatotoxicity. In this study the effects of MDMA and its metabolites on the glutathione system were evaluated. Glutathione (GSH/GSSG) levels and gene expression of glutamate cysteine ligase catalytic subunit (GCLC), glutathione-S-transferase (GST) and pregnane X receptor (PXR) were compared in the immortalized human liver epithelial cell line THLE-Neo lacking phase I metabolism and primary rat hepatocytes expressing both phase I and II metabolism. Furthermore, we evaluated the potential protective effects of two antioxidants, N-acetyl-cysteine (NAC) and sulforaphane (SFN) in these cell systems. In THLE-Neo cells, the MDMA metabolite 3,4-dihydroxymetamphetamine (HHMA) significantly decreased cell viability and depleted GSH levels, resulting in an increased expression of GCLC and GST up to 3.4- and 2.2-fold, respectively. In primary rat hepatocytes, cell viability or GSH levels were not significantly affected upon MDMA exposure. GCLC expression levels where not significantly altered either, although GST expression was increased 2.3-fold. NAC counteracted MDMA-induced cytotoxicity and restored GSH levels. Phase II enzyme expression was also reverted. Conversely, SFN increased MDMA-induced cytotoxicity and GSH depletion, while GCLC and GST expression were significantly induced. In addition, PXR expression decreased after HHMA and MDMA exposure, while co-exposure to SFN induced it up to 3.6- and 3.9-fold compared to vehicle-control in the THLE-Neo cells and rat hepatocytes, respectively. Taken together, these data indicate that HHMA is a major factor in the MDMA-mediated hepatotoxicity through interaction with the glutathione system. The results of our study show that for MDMA intoxication the treatment with an antioxidant such as NAC may counteract the potentially hepatotoxicity. However, SFN supplementation should be considered with care because of the indications of possible drug-drug interactions.
PMID:21871945 Antolino-Lobo I et al; Toxicology 289 (2-3): 175-84 (2011)
3,4-Methylenedioxymethamphetamine (MDMA; ecstasy) is a ring-substituted amphetamine widely used for recreational purposes. MDMA is predominantly O-demethylenated in humans by cytochrome P450 (CYP) 2D6, and is also a potent mechanism-based inhibitor of the enzyme. After assessing the inhibition and recovery of CYP2D6 in a previous study, the aim of this work was to study in humans the activity of CYP1A2 in vivo after CYP2D6 had been inhibited by MDMA, using caffeine as a probe drug. Twelve male and nine female recreational MDMA users were included. In session 1, 100 mg of caffeine was given at 0 hr. In session 2, a 1.5 mg/kg MDMA dose (range 75-100 mg) was given at 0 hr followed by a 100 mg dose of caffeine 4 hr later. Aliquots of plasma were assayed for caffeine (137X) and paraxanthine (17X) and statistically significant differences were assessed with a one-way ANOVA. There were significant gender differences at basal condition, which persisted after MDMA administration. CYP1A2 activity was higher in both genders after drug administration, with an increase in 40% in females and 20% in males. Results show an increase in CYP1A2 activity when CYP2D6 is inhibited by MDMA in both genders, being more pronounced in females.
PMID:22673010 Yubero-Lahoz S et al; Drug Metab Pharmacokinet 27 (6): 605-13 (2012)
The R- and S-enantiomers of racemic 3,4-methylenedioxymethamphetamine (MDMA) exhibit different dose-concentration curves. In plasma, S-MDMA was eliminated at a higher rate, most likely due to stereoselective metabolism. Similar data were shown in various in vitro experiments. The aim of the present study was the in vivo investigation of stereoselective elimination of MDMA's phase I and phase II metabolites in human urine following controlled oral MDMA administration. Urine samples from 10 participants receiving 1.0 and 1.6 mg/kg MDMA separated by at least one week were analyzed blind by liquid chromatography-high resolution-mass spectrometry and gas chromatography-mass spectrometry after chiral derivatization with S-heptafluorobutyrylprolyl chloride. R/S ratios at C(max) were comparable after low and high doses with ratios >1 for MDMA, free 3,4-dihydroxymeth-amphetamine (DHMA), and 4-hydroxy-3-methoxymethamphetamine (HMMA) sulfate, and with ratios <1 for 3,4-methylendioxyamphetamine (MDA), free HMMA, DHMA sulfate and HMMA glucuronide. In the five days after the high MDMA dose, a median of 21% of all evaluated compounds were excreted as R-stereoisomers and 17% as S-stereoisomers. Significantly greater MDMA, DHMA, and HMMA sulfate R-enantiomers and HMMA and HMMA glucuronide S-stereoisomers were excreted. No significant differences were observed for MDA and DHMA sulfate stereoisomers. Changes in R/S ratios could be observed over time for all analytes, with steady increases in the first 48 hr. R/S ratios could help to roughly estimate time of MDMA ingestion and therefore, improve interpretation of MDMA and metabolite urinary concentrations in clinical and forensic toxicology.
PMID:21983032 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3225738 Schwaninger AE et al; Biochem Pharmacol 83 (1): 131-8 (2012)
3,4-Methylenedioxy-amphetamine (MDA) and benzodioxolyl-butanamine (BDB) are chiral designer drugs distributed on the illicit drug market and they are also N-dealkyl metabolites of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy, Adam), 3,4-methylenedioxyethylamphetamine (MDEA, Eve), and N-methyl-benzodioxolyl-butanamine (MBDB, Eden), respectively. MDA and BDB are mainly metabolized via demethylenation to the corresponding catecholamines. The aim of the present work was to elucidate the contribution of the relevant human P450s in the demethylenation of the MDA and BDB enantiomers. They were incubated using heterologously expressed human P450s and the corresponding metabolites dihydroxyamphetamine and 1,2-dihydroxy-4-[2-amino-butyl]benzene were determined. Highest contributions to the demethylenation as calculated from the enzyme kinetic data were obtained for CYP2D6 (MDA and BDB) and additionally CYP3A4 in the case of BDB at substrate concentrations corresponding to plasma concentrations of recreational users. A preferred transformation of the S-enantiomer could be observed for the CYP2D6- and CYP3A4-catalyzed reactions.
PMID:19576971 Meyer MR et al; Toxicol Lett 190 (1): 54-60 (2009)
For more Metabolism/Metabolites (Complete) data for 3,4-Methylenedioxymethamphetamine (18 total), please visit the HSDB record page.
610 (though duration of effects is typically actually 35 hours)
The present study compared the disposition and metabolism of the recreational drug (+/-) 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") in squirrel monkeys and humans because the squirrel monkey has been extensively studied for MDMA neurotoxicity. ... The elimination half-life of MDMA was considerably shorter in squirrel monkeys than in humans (2-3 versus 6-9 hours). ...
PMID:19417716 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3159867 Mueller M et al; Ther Drug Monit 31 (3): 367-73 (2009)
... In this study, we examine MDMA pharmacokinetics in rats given low (2 mg/kg) and high (10 mg/kg) doses of racemic MDMA via intraperitoneal, subcutaneous, and oral routes. Repeated blood specimens were collected from venous catheters, and plasma was assayed for MDMA and its metabolites, 4-hydroxy-3-methoxymethamphetamine (HMMA) and 3,4-methylenedioxyamphetamine (MDA), by gas chromatography-mass spectrometry. ... MDMA plasma half-lives were <1 hr for low-dose groups, whereas HMMA and MDA half-lives were >2 hr. ...
PMID:19679675 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774984 Baumann MH et al; Drug Metab Dispos 37 (11): 2163-70 (2009)
... Elimination half-life was 8.6 hr /(75 mg)/ and 7.7 hr /(125 mg)/ for low and high dose 3,4-methylenedioxymethamphetamine /in eight men with experience in the recreational use of 3,4-methylenedioxymethamphetamine/ ...
PMID:10381769 Mas M et al; J Pharmacol Exp Ther 290 (1): 136-45 (1999)
... /The/ half-life of (R)-3,4-methylenedioxymethamphetamine (5.8 +/- 2.2 hr) was significantly longer than that of the S-enantiomer (3.6 +/- 0.9 hr). ...
PMID:10388483 Fallon JK et al; Clinical Chemistry 45 (7): 1058-69 (1999)
Studies to characterize the pharmacokinetics of the enantiomers of MDMA were conducted in rats using the iliac arterial cannulation. Two routes of administration, intravenous and subcutaneous, were evaluated at two dose levels for each route [20 and 40 mg/kg (+/-)-MDMA for subcutaneous, 10 and 20 mg/kg (+/-)-MDMA for intravenous administrations]. The average half-life (+/- SD) for all dosing groups was 2.5 +/- 0.8 hr for (-)-(R)-MDMA and 2.2 +/- 0.8 hr for (+)-(S)-MDMA. ...
PMID:2083146 Fitzgerald RL et al; Chirality 2 (4): 241-8 (1990)
It enters neurons via carriage by the monoamine transporters. Once inside, MDMA inhibits the vesicular monoamine transporter, which results in increased concentrations of serotonin, norepinephrine, and dopamine into the cytoplasm, and induces their release by reversing their respective transporters through a process known as phosphorylation. It also acts as a weak 5-HT1 and 5-HT2 receptor agonist. MDMA's unusual entactogenic effects have been hypothesized to be, at least partly, the result of indirect oxytocin secretion via activation of the serotonin system. Oxytocin is a hormone released following events like hugging, orgasm, and childbirth, and is thought to facilitate bonding and the establishment of trust. Based on studies in rats, MDMA is believed to cause the release of oxytocin, at least in part, by both directly and indirectly agonizing the serotonin 5-HT1A receptor.
3,4-Methylenedioxymethamphetamine (MDMA; "ecstasy") is a potentially neurotoxic recreational drug of abuse. Though the mechanisms involved are still not completely understood, formation of reactive metabolites and mitochondrial dysfunction contribute to MDMA-related neurotoxicity. Neuronal mitochondrial trafficking, and their targeting to synapses, is essential for proper neuronal function and survival, rendering neurons particularly vulnerable to mitochondrial dysfunction. Indeed, MDMA-associated disruption of Ca(2+) homeostasis and ATP depletion have been described in neurons, thus suggesting possible MDMA interference on mitochondrial dynamics. In this study, we performed real-time functional experiments of mitochondrial trafficking to explore the role of in situ mitochondrial dysfunction in MDMA's neurotoxic actions. We show that the mixture of MDMA and six of its major in vivo metabolites, each compound at 10uM, impaired mitochondrial trafficking and increased the fragmentation of axonal mitochondria in cultured hippocampal neurons. Furthermore, the overexpression of mitofusin 2 (Mfn2) or dynamin-related protein 1 (Drp1) K38A constructs almost completely rescued the trafficking deficits caused by this mixture. Finally, in hippocampal neurons overexpressing a Mfn2 mutant, Mfn2 R94Q, with impaired fusion and transport properties, it was confirmed that a dysregulation of mitochondrial fission/fusion events greatly contributed to the reported trafficking phenotype. In conclusion, our study demonstrated, for the first time, that the mixture of MDMA and its metabolites, at concentrations relevant to the in vivo scenario, impaired mitochondrial trafficking and increased mitochondrial fragmentation in hippocampal neurons, thus providing a new insight in the context of "ecstasy"-induced neuronal injury.
PMID:24595818 Barbosa DJ et al; Toxicol Sci 139 (2): 407-20 (2014)
The aim of the present study was to identify the neural substrate underlying memory impairment due to a single dose of MDMA (3,4-methylenedioxymethamphetamine) by means of pharmaco-MRI. Based on previous behavioral results it was hypothesized that this deficit could be attributed to a specific influence of MDMA on encoding. Fourteen Ecstasy users participated in this double-blind, placebo-controlled, within-subject study with two treatment conditions: MDMA (75 mg) and placebo. Memory performance was tested by means of a word learning task including two words lists, one addressing reading processes (control task, CWL) and a second (experimental task, EWL) addressing encoding and reading processes. Behavioral data showed that under the influence of MDMA, EWL performance was worse than placebo. Imaging data showed that Encoding was situated mainly in (pre)frontal, temporal and parietal areas. MDMA by Encoding interaction was situated in three areas: the left middle frontal gyrus (BA10), the right fusiform gyrus (BA19), and the left cuneus (BA18). Behavioral and functional data only correlated in BA10. It appeared that EWL performance caused BOLD signal change in BA10 during placebo treatment but not during MDMA intoxication. It is concluded that MDMA influences middle frontal gyrus processes resulting in impoverished memory encoding.
PMID:21616977 Kuypers KP et al; J Psychopharmacol 25 (8): 1053-61 (2011)
The recreational drug 3,4-methylenedioxymethamphetamine (MDMA; 'ecstasy') produces a neuro-inflammatory response in rats characterized by an increase in microglial activation and IL-1beta levels. The integrity of the blood-brain barrier (BBB) is important in preserving the homeostasis of the brain and has been shown to be affected by neuro-inflammatory processes. We aimed to study the effect of a single dose of MDMA on the activity of metalloproteinases (MMPs), expression of extracellular matrix proteins, BBB leakage and the role of the ionotropic purinergic receptor P2X7 (P2X7R) in the changes induced by the drug. Adult male Dark Agouti rats were treated with MDMA (10 mg/kg, i.p.) and killed at several time-points in order to evaluate MMP-9 and MMP-3 activity in the hippocampus and laminin and collagen-IV expression and IgG extravasation in the dentate gyrus. Microglial activation, P2X7R expression and localization were also determined in the dentate gyrus. Separate groups were treated with MDMA and the P2X7R antagonists Brilliant Blue G (BBG; 50 mg/kg, i.p.) or A-438079 (30 mg/kg, i.p.). MDMA increased MMP-3 and MMP-9 activity, reduced laminin and collagen-IV expression and increased IgG immunoreactivity. In addition, MDMA increased microglial activation and P2X7R immunoreactivity in these cells. BBG suppressed the increase in MMP-9 and MMP-3 activity, prevented basal lamina degradation and IgG extravasation into the brain parenchyma. A-438079 also prevented the MDMA-induced reduction in laminin and collagen-IV immunoreactivity. These results indicate that MDMA alters BBB permeability through an early P2X7R-mediated event, which in turn leads to enhancement of MMP-9 and MMP-3 activity and degradation of extracellular matrix.
PMID:24626059 Rubio-Araiz A et al; Int J Neuropsychopharmacol 17 (8): 1243-55 (2014)
3,4-Methylenedioxymethamphetamine (MDMA) produces a neuroinflammatory reaction in rat brain characterized by an increase in interleukin-1 beta (IL-1beta) and microglial activation. The CB2 receptor agonist JWH-015 reduces both these changes and partially protects against MDMA-induced neurotoxicity. We have examined MDMA-induced changes in IL-1 receptor antagonist (IL-1ra) levels and IL-1 receptor type I (IL-1RI) expression and the effects of JWH-015. The cellular location of IL-1beta and IL-1RI was also examined. MDMA-treated animals were given the soluble form of IL-1RI (sIL-1RI) and neurotoxic effects examined. Dark Agouti rats received MDMA (12.5 mg/kg, i.p.) and levels of IL-1ra and expression of IL-1RI measured 1 hr, 3 hr or 6 hr later. JWH-015 (2.4 mg/kg, i.p.) was injected 48 hr, 24 hr and 0.5 hr before MDMA and IL-1ra and IL-1RI measured. For localization studies, animals were sacrificed 1 hr or 3 hr following MDMA and stained for IL-1beta or IL-1RI in combination with neuronal and microglial markers. sIL-1RI (3 ug/animal; i.c.v.) was administered 5 min before MDMA and 3 hr later. 5-HT transporter density was determined 7 days after MDMA injection. MDMA produced an increase in IL-ra levels and a decrease in IL-1RI expression in hypothalamus which was prevented by CB2 receptor activation. IL-1RI expression was localized on neuronal cell bodies while IL-1beta expression was observed in microglial cells following MDMA. sIL-1RI potentiated MDMA-induced neurotoxicity. MDMA also increased IgG immunostaining indicating that blood brain-barrier permeability was compromised. In summary, MDMA produces changes in IL-1 signal modulators which are modified by CB2 receptor activation. These results indicate that IL-1beta may play a partial role in MDMA-induced neurotoxicity.
PMID:21595923 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3113340 Torres E et al; J Neuroinflammation 8: 53 (2011)
For more Mechanism of Action (Complete) data for 3,4-Methylenedioxymethamphetamine (9 total), please visit the HSDB record page.
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MDMA (Midomafetamine) is a ring-substituted amphetamine analog with reinforcing psychoactive effects. It is a 5-HT2A receptor agonist, which is being evaluated for the treatment of PTSD.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: MDMA
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Lykos Therapeutics Reorganizes After FDA Decision on Midomafetamine Capsules for PTSD
Details : MDMA (Midomafetamine) is a ring-substituted amphetamine analog with reinforcing psychoactive effects. It is a 5-HT2A receptor agonist, which is being evaluated for the treatment of PTSD.
Brand Name : MDMA
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MDMA (Midomafetamine) is a ring-substituted amphetamine analog with reinforcing psychoactive effects. It is a 5-HT2A receptor agonist, FDA PDAC voted against for its use PTSD treatment.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: MDMA
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Lykos Therapeutics Provides Update on FDA Advisory Meeting for MDMA-Assisted PTSD Therapy
Details : MDMA (Midomafetamine) is a ring-substituted amphetamine analog with reinforcing psychoactive effects. It is a 5-HT2A receptor agonist, FDA PDAC voted against for its use PTSD treatment.
Brand Name : MDMA
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Net proceeds will support development of novel intellectual property assets, including ALA-002 (3,4-methylenedioxymethamphetamine) for treating PTSD and social anxiety in autistic adults.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: ALA-002
Study Phase: Phase IProduct Type: Small molecule
Sponsor: Undisclosed
Deal Size: $0.7 million Upfront Cash: Undisclosed
Deal Type: Private Placement April 19, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : $0.7 million
Deal Type : Private Placement
PharmAla Closes Private Placement and Concurrent Debt Settlement
Details : Net proceeds will support development of novel intellectual property assets, including ALA-002 (3,4-methylenedioxymethamphetamine) for treating PTSD and social anxiety in autistic adults.
Brand Name : ALA-002
Molecule Type : Small molecule
Upfront Cash : Undisclosed
April 19, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Net proceeds will support development of novel intellectual property assets, including ALA-002 (3,4-methylenedioxymethamphetamine) for treating PTSD and social anxiety in autistic adults.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: ALA-002
Study Phase: PreclinicalProduct Type: Small molecule
Sponsor: Undisclosed
Deal Size: $0.7 million Upfront Cash: Undisclosed
Deal Type: Private Placement April 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Preclinical
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : $0.7 million
Deal Type : Private Placement
PharmAla Announces Private Placement and Concurrent Debt Settlement
Details : Net proceeds will support development of novel intellectual property assets, including ALA-002 (3,4-methylenedioxymethamphetamine) for treating PTSD and social anxiety in autistic adults.
Brand Name : ALA-002
Molecule Type : Small molecule
Upfront Cash : Undisclosed
April 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MDMA (Midomafetamine) is a ring-substituted amphetamine analog with reinforcing psychoactive effects. It is a 5-HT2A receptor agonist, which is under development for posttraumatic stress disorder.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: MDMA
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 09, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Lykos' MDMA-Assisted PTSD Therapy Receives NDA Acceptance
Details : MDMA (Midomafetamine) is a ring-substituted amphetamine analog with reinforcing psychoactive effects. It is a 5-HT2A receptor agonist, which is under development for posttraumatic stress disorder.
Brand Name : MDMA
Molecule Type : Small molecule
Upfront Cash : Not Applicable
February 09, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Funds support regulatory activities for MDMA-assisted PTSD therapy combining MDMA with psychological care.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: MDMA-assisted Therapy
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Helena
Deal Size: $100.0 million Upfront Cash: Undisclosed
Deal Type: Series A Financing January 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Helena
Deal Size : $100.0 million
Deal Type : Series A Financing
Maps Public Benefit Corporation Announces Oversubscribed Series A Financing Renamed Lykos
Details : Funds support regulatory activities for MDMA-assisted PTSD therapy combining MDMA with psychological care.
Brand Name : MDMA-assisted Therapy
Molecule Type : Small molecule
Upfront Cash : Undisclosed
January 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MDMA (3,4-methylenedioxymethamphetamine) is a synthetic, psychoactive drug with a chemical structure similar to the stimulant methamphetamine and the hallucinogen mescaline being developed the treatment of for PTSD.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: MDMA
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
MAPS Celebrates Submission of New Drug Application to FDA for MDMA-Assisted Therapy for PTSD
Details : MDMA (3,4-methylenedioxymethamphetamine) is a synthetic, psychoactive drug with a chemical structure similar to the stimulant methamphetamine and the hallucinogen mescaline being developed the treatment of for PTSD.
Brand Name : MDMA
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MDMA (3,4-methylenedioxymethamphetamine) is a 5-HT2A receptor agonist, small molecule drug candidate, which is currently being evaluated for the treatment of patients with Post-traumatic stress disorder.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: MDMA
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 12, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : MDMA (3,4-methylenedioxymethamphetamine) is a 5-HT2A receptor agonist, small molecule drug candidate, which is currently being evaluated for the treatment of patients with Post-traumatic stress disorder.
Brand Name : MDMA
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 12, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MDMA (3,4-methylenedioxymethamphetamine) is a synthetic, psychoactive drug with a chemical structure similar to the stimulant methamphetamine and the hallucinogen mescaline.
Lead Product(s): Midomafetamine
Therapeutic Area: Oncology Brand Name: MDMA
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 03, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Sunstone Therapies Announces Expansion of Innovative Dyad Study
Details : MDMA (3,4-methylenedioxymethamphetamine) is a synthetic, psychoactive drug with a chemical structure similar to the stimulant methamphetamine and the hallucinogen mescaline.
Brand Name : MDMA
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 03, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MDMA-AT (3,4-methylenedioxymethamphetamine) mainly acts as a releaser of serotonin (5-HT) and noradrenaline, and to a lesser extent also of dopamine, which is investigated for the treatment of Posttraumatic Stress Disorder.
Lead Product(s): Midomafetamine
Therapeutic Area: Psychiatry/Psychology Brand Name: MDMA-AT
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 14, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Midomafetamine
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : MDMA-AT (3,4-methylenedioxymethamphetamine) mainly acts as a releaser of serotonin (5-HT) and noradrenaline, and to a lesser extent also of dopamine, which is investigated for the treatment of Posttraumatic Stress Disorder.
Brand Name : MDMA-AT
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 14, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?