
Synopsis
Synopsis
0
EU WC
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
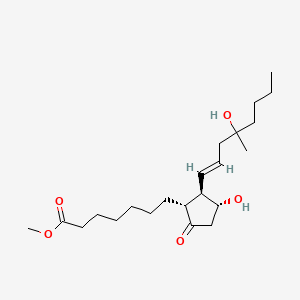

1. Apo Misoprostol
2. Apo-misoprostol
3. Cytotec
4. Glefos
5. Misoprostol, (11alpha,13e)-isomer
6. Misoprostol, (11alpha,13e,16r)-isomer
7. Misoprostol, (11alpha,13z)-(+-)-isomer
8. Misoprostol, (11alpha.13e,16s)-isomer
9. Misoprostol, (11beta,13e)-(+-)-isomer
10. Misoprostol, (11beta,13e,16r)-isomer
11. Misoprostol, (11beta,13e,16s)-isomer
12. Novo Misoprostol
13. Novo-misoprostol
14. Sc 29333
15. Sc 30249
16. Sc-29333
17. Sc-30249
18. Sc29333
19. Sc30249
1. 59122-46-2
2. Cytotec
3. Isprelor
4. Sc-29333
5. Misoprostolum [inn-latin]
6. Misodel
7. Sc 29333
8. Ccris 6859
9. Hsdb 3573
10. Methyl 7-[(1r,2r,3r)-3-hydroxy-2-[(e)-4-hydroxy-4-methyloct-1-enyl]-5-oxocyclopentyl]heptanoate
11. Brn 4155643
12. 103601-27-0
13. 0e43v0bb57
14. Prost-13-en-1-oic Acid, 11,16-dihydroxy-16-methyl-9-oxo-, Methyl Ester,(11a,13e)-
15. Ncgc00162445-02
16. Misoprostolum
17. Misoprost
18. Misotol
19. Misogon
20. Misopess
21. Misotac
22. Gymiso
23. Methyl (11alpha,13e)-11,16-dihydroxy-16-methyl-9-oxoprost-13-en-1-oate
24. 7-[(1r,2r,3r)-3-hydroxy-2-[(e)-4-hydroxy-4-methyloct-1-enyl]-5-oxocyclopentyl]heptanoic Acid Methyl Ester
25. Cytotec (tn)
26. Misoprostol (methyl Ester)
27. Sr-01000695425
28. Unii-0e43v0bb57
29. Xp-16j
30. Mvi 200
31. Methyl 7-((1r,2r,3r)-3-hydroxy-2-((e)-4-hydroxy-4-methyloct-1-enyl)-5-oxocyclopentyl)heptanoate
32. 11
33. A-misoprostol
34. Cas_59122-46-2
35. Misoprostol [usan:usp:inn:ban:jan]
36. Misoprostol [mi]
37. Dsstox_cid_897
38. Misoprostol [inn]
39. Misoprostol [jan]
40. Methyl (+-)-11-alpha,16-dihydroxy-16-methyl-9-oxoprost-13-en-1-oate
41. Misoprostol [hsdb]
42. Misoprostol [usan]
43. Chembl606
44. Misoprostol [vandf]
45. Schembl7787
46. (11-alpha,13e)-(+-)-11,16-dihydroxy-16-methyl-9-oxoprost-13-en-1-oic Acid Methyl Ester
47. Dsstox_rid_75852
48. Misoprostol [mart.]
49. Dsstox_gsid_20897
50. Misoprostol [usp-rs]
51. Misoprostol [who-dd]
52. Mls000028863
53. Misoprostol (jan/usp/inn)
54. Misoprostol, 1% In Cellulose
55. Gtpl1936
56. Dtxsid7020897
57. Bdbm85606
58. Chebi:94387
59. Misoprostol, >=99% (hplc)
60. Misoprostol [orange Book]
61. Regid_for_cid_5282381
62. Hms2090l10
63. Hms3648f03
64. Hms3715k08
65. Misoprostol [ep Monograph]
66. Misoprostol [usp Monograph]
67. Ex-a1774
68. Hy-b0610
69. Tox21_112010
70. Arthrotec Component Misoprostol
71. Akos015899652
72. Ccg-221093
73. Db00929
74. Prost-13-en-1-oic Acid, 11,16-dihydroxy-16-methyl-9-oxo-, Methyl Ester, (11alpha,13e)-
75. Smp1_000193
76. Misoprostol Component Of Arthrotec
77. Ncgc00162445-01
78. Ncgc00162445-03
79. (+-)-methyl (1r,2r,3r)-3-hydroxy-2-((e)-(4rs)-4-hydroxy-4-methyl-1-octenyl)-5-oxocyclopentaneheptanoate
80. 62015-39-8
81. As-83017
82. Smr000058558
83. Cas-59122-46-2
84. D00419
85. Ab00513745-05
86. 122m462
87. A832173
88. Q416025
89. Q-201409
90. Sr-01000695425-2
91. Sr-01000695425-4
92. Brd-a50310035-001-01-6
93. Misoprostol, European Pharmacopoeia (ep) Reference Standard
94. Misoprostol, United States Pharmacopeia (usp) Reference Standard
95. (+/-) Methyl 11alpha, 16-dihydroxy-16-methyl-9-oxoprost-13e-en-1-oate
96. (+/-) Methyl 11alpha,16-dihydroxy-16-methyl-9-oxoprost-13e-en-1-oate
97. (+/-) Methyl-11alpha,16-dihydroxy-16-methyl-9-oxoprost-13e-en-1-oate
98. (+/-) Methyl-11alpha,16-dihydroxy-16-methyl-9-oxoprost13e-en-1-oate
99. (+/-)-(11a,13e)-11,16-dihydroxy-16-methyl-9-oxo-prost-13-en-1-oic Acid Methyl Ester
100. (11alpha,13e)-11,16-dihydroxy-16-methyl-9-oxoprosta-13-ene-1-oic Acid Methyl Ester
101. 9-oxo-11alpha,16-dihydroxy-16-methyl-prost-13e-en-1-oic Acid, Methyl Ester
102. Misoprostol For System Suitability, European Pharmacopoeia (ep) Reference Standard
103. (+/-)-methyl (1r,2r,3r)-3-hydroxy-2-((e)-(4rs)-4-hydroxy-4-methyl-1-octenyl)-5-oxocyclopentaneheptanoate
104. Methyl 7-[(1r,2r,3r)-2-[(e)-4-methyl-4-oxidanyl-oct-1-enyl]-3-oxidanyl-5-oxidanylidene-cyclopentyl]heptanoate
105. Methyl 7-[(1r,2r,3r)-3-hydroxy-2-[(1e)-4-hydroxy-4-methyloct-1-en-1-yl]-5-oxocyclopentyl]heptanoate
106. Prost-13-en-1-oic Acid, 11,16-dihydroxy-16-methyl-9-oxo-, Methyl Ester, (11.alpha.,13e)-(+/-)-
107. Prost-13-en-1-oic Acid, 11,16-dihydroxy-16-methyl-9-oxo-, Methyl Ester, (11alpha,13e)-(+-)-
108. Rel-methyl 7-((1r,2r,3r)-3-hydroxy-2-((e)-4-hydroxy-4-methyloct-1-en-1-yl)-5-oxocyclopentyl)heptanoate
| Molecular Weight | 382.5 g/mol |
|---|---|
| Molecular Formula | C22H38O5 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 14 |
| Exact Mass | 382.27192431 g/mol |
| Monoisotopic Mass | 382.27192431 g/mol |
| Topological Polar Surface Area | 83.8 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 487 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Cytotec |
| PubMed Health | Misoprostol (By mouth) |
| Drug Classes | Antiulcer, Protectant, Endocrine-Metabolic Agent |
| Drug Label | Misoprostol oral tablets contain either 100 mcg or 200 mcg of misoprostol, a synthetic prostaglandin E1 analog.Misoprostol contains approximately equal amounts of the two diastereomers presented below with their enantiomers indicated by ():Misopros... |
| Active Ingredient | Misoprostol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.2mg; 0.1mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 2 of 4 | |
|---|---|
| Drug Name | Misoprostol |
| PubMed Health | Misoprostol (By mouth) |
| Drug Classes | Antiulcer, Protectant, Endocrine-Metabolic Agent |
| Drug Label | Misoprostol oral tablets contain either 100 mcg or 200 mcg of misoprostol, a synthetic prostaglandin E1 analog.Misoprostol contains approximately equal amounts of the two diastereomers presented below with their enantiomers indicated by ():Misopros... |
| Active Ingredient | Misoprostol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.1mg; 0.2mg |
| Market Status | Prescription |
| Company | Novel Labs; Ivax Sub Teva Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Cytotec |
| PubMed Health | Misoprostol (By mouth) |
| Drug Classes | Antiulcer, Protectant, Endocrine-Metabolic Agent |
| Drug Label | Misoprostol oral tablets contain either 100 mcg or 200 mcg of misoprostol, a synthetic prostaglandin E1 analog.Misoprostol contains approximately equal amounts of the two diastereomers presented below with their enantiomers indicated by ():Misopros... |
| Active Ingredient | Misoprostol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.2mg; 0.1mg |
| Market Status | Prescription |
| Company | Gd Searle |
| 4 of 4 | |
|---|---|
| Drug Name | Misoprostol |
| PubMed Health | Misoprostol (By mouth) |
| Drug Classes | Antiulcer, Protectant, Endocrine-Metabolic Agent |
| Drug Label | Misoprostol oral tablets contain either 100 mcg or 200 mcg of misoprostol, a synthetic prostaglandin E1 analog.Misoprostol contains approximately equal amounts of the two diastereomers presented below with their enantiomers indicated by ():Misopros... |
| Active Ingredient | Misoprostol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.1mg; 0.2mg |
| Market Status | Prescription |
| Company | Novel Labs; Ivax Sub Teva Pharms |
Abortifacient Agents, Nonsteroidal; Anti-Ulcer Agents; Oxytocics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Misoprostol is indicated for the prevention of gastric ulcer associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, in patients at high risk of complications from gastric ulcer, such as the elderly, and in patients with concomitant disease or patients at high risk of developing gastric ulceration, such as those with a history of ulcer. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2085
Misoprostol is indicated in the short-term treatment of duodenal ulcer. /NOT included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2085
The efficacy and tolerability of mifepristone in combo with misoprostol for termination of early pregnancy (up to 49 days of amenorrhea) are established.
PMID:8574255 Aub'eny E et al; Int J Fertil Menopausal Stud 40 (Suppl 2): 85-91 (1995)
For more Therapeutic Uses (Complete) data for MISOPROSTOL (8 total), please visit the HSDB record page.
Misoprostol is contraindicated during pregnancy. Studies in humans have shown that misoprostol causes an increase in the frequency and intensity of uterine contractions. Misoprostol administration has also been associated with a higher incidence of uterine bleeding and expulsion of uterine contents. Miscarriages caused by misoprostol are likely to be incomplete, resulting in very serious medical complications, sometimes requiring hospitalization and surgery, and possibly causing infertility.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2086
Patients of childbearing potential may use misoprostol if nonsteroidal anti-inflammatory drug (NSAID) therapy is required and patient is at high risk of complications from gastric ulcers associated with the use of NSAIDs, or is at high risk of developing gastric ulceration. Such patients must comply with effective contraceptive measures, must have had a negative serum pregnancy test within 2 weeks prior to initiation of therapy and must start misoprostol therapy only on the second or third day of the next normal menstrual period.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2086
It is unlikely that misoprostol is distributed into breast milk since it is rapidly metabolized throughout the body. however, it is not known if the active metabolite, misoprostol acid, is distributed into breast milk. Therefore, administration of misoprostol to nursing women is not recommended because of the potential distribution of misoprostol acid, which could cause significant diarrhea in the nursing infant.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2086
Misoprostol generally is well tolerated. The frequency of adverse effects does not appear to be affected by patient age in adults. The most frequent adverse effects associated with misoprostol therapy involve the GI tract (e.g., diarrhea, nausea, abdominal pain).
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2169
For more Drug Warnings (Complete) data for MISOPROSTOL (8 total), please visit the HSDB record page.
Misoprostol is indicated as a tablet to reduce the risk of NSAID induced gastric ulcers but not duodenal ulcers in high risk patients. Misoprostol is also formulated in combination with diclofenac to treat symptoms of osteoarthritis or rheumatoid arthritis in patients with a high risk of developing gastric ulcers. Misoprostol is used off label for the management of miscarriages, prevention of post partum hemorrhage, and is also used alone or in combination with mifepristone in other countries for first trimester abortions.
FDA Label
Induction of labour
Induction of labour
Misoprostol is a prostaglandin E1 analog used to reduce the risk of NSAID induced gastric ulcers by reducing secretion of gastric acid from parietal cells. Misoprostol is also used to manage miscarriages and used alone or in combination with mifepristone for first trimester abortions. An oral dose of misoprostol has an 8 minute onset of action and a duration of action of approximately 2 hours, a sublingual dose has an 11 minute onset of action and a duration of action of approximately 3 hours, a vaginal dose has a 20 minute onset of action and a duration of action of approximately 4 hours, and a rectal dose has a 100 minute onset of action and a duration of action of approximately 4 hours.
Oxytocics
Drugs that stimulate contraction of the myometrium. They are used to induce LABOR, OBSTETRIC at term, to prevent or control postpartum or postabortion hemorrhage, and to assess fetal status in high risk pregnancies. They may also be used alone or with other drugs to induce abortions (ABORTIFACIENTS). Oxytocics used clinically include the neurohypophyseal hormone OXYTOCIN and certain prostaglandins and ergot alkaloids. (From AMA Drug Evaluations, 1994, p1157) (See all compounds classified as Oxytocics.)
Abortifacient Agents, Nonsteroidal
Non-steroidal chemical compounds with abortifacient activity. (See all compounds classified as Abortifacient Agents, Nonsteroidal.)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BB - Prostaglandins
A02BB01 - Misoprostol
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02A - Uterotonics
G02AD - Prostaglandins
G02AD06 - Misoprostol
Absorption
For an 800g oral dose of misoprostol, the AUC was 2.01920.8032h\*ng/mL, the Cmax was 2.68301.2161ng/mL, and a tmax of 0.3450.186h. For a 800g sublingual dose of misoprostol, the AUC was 3.20941.0417h\*ng/mL, the Cmax was 2.43911.1567ng/mL, and a tmax of 0.7120.415h. For a 800g buccal dose of misoprostol, the AUC was 2.07260.3578h\*ng/mL, the Cmax was 1.36110.3436ng/mL, and a tmax of 1.3080.624h.
Route of Elimination
As much as 73.24.6% of a radiolabelled oral dose of misoprostol is recovered in the urine.
Volume of Distribution
Data regarding the volume of distribution of misoprostol is scarce. The apparent volume of distribution of the active metabolite of misoprostol was in subjects with normal renal function was 13.68.0L/kg, with mild renal impairment was 17.323.0L/kg, with moderate renal impairment was 14.36.8L/kg, and with end stage renal disease was 11.09.6L/kg.
Clearance
Because of the rapid de-esterification of misoprostol before or during absorption, it is usually undetectable in plasma. Misoprostol's active metabolite, misoprostol acid, has a total body clearance of 0.286L/kg/min. Subjects with mild renal impairment had a total body clearance of 0.2260.073L/kg/min, subjects with moderate renal impairment had a total body clearance of 0.2700.103L/kg/min, and subjects with end stage renal disease had a total body clearance of 0.1050.052L/kg/min.
Rapidly absorbed following oral administration.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2085
Elimination: Renal (64 to 73% of the oral dose excreted within the first 24 hours). Fecal (15% of the oral dose).
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2086
Misoprostol is de-esterified to its active metabolite, misoprostol acid, also known as SC-30695. This metabolite is further reduced to dinor and tetranor metabolites (SC-41411), a prostaglandin F1 (PGF1) analog of SC-41411, and a -16-carboxylic acid derivative. However, the majority of these metabolites are not well described in the literature.
Rapidly de-esterified to misoprostol acid (primary biologically active metabolite). The de-esterified metabolite undergoes further metabolism by beta and omega oxidation, which can take place in various tissues in the body.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2085
The half life of an 800g oral dose is 1.04010.5090h, for a sublingual dose is 0.85420.1170h, and for a buccal dose is 0.83650.1346h.
Terminal - 20-40 minutes
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2085
Misoprostol is a synthetic prostaglandin E1 analog that stimulates prostaglandin E1 receptors on parietal cells in the stomach to reduce gastric acid secretion. Mucus and bicarbonate secretion are also increased along with thickening of the mucosal bilayer so the mucosa can generate new cells. Misoprostol binds to smooth muscle cells in the uterine lining to increase the strength and frequency of contractions as well as degrade collagen and reduce cervical tone.
Misoprostol enhances natural gastromucosal defense mechanisms and healing in acid-related disorders, probably by increasing production of gastric mucus and mucosal secretion of bicarbonate.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2085
Misoprostol inhibits basal and nocturnal gastric acid secretion by direct action on the parietal cells; also inhibits gastric acid secretion stimulated by food, histamine, and pentagastrin. It decreases pepsin secretion under basal, but not histamine stimulation. Misoprostol has no significant effect on fasting or postprandial gastrin or intrinsic factor output.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2085

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
17
PharmaCompass offers a list of Misoprostol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Misoprostol manufacturer or Misoprostol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Misoprostol manufacturer or Misoprostol supplier.
PharmaCompass also assists you with knowing the Misoprostol API Price utilized in the formulation of products. Misoprostol API Price is not always fixed or binding as the Misoprostol Price is obtained through a variety of data sources. The Misoprostol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Misoprostol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Misoprostol, including repackagers and relabelers. The FDA regulates Misoprostol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Misoprostol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Misoprostol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Misoprostol supplier is an individual or a company that provides Misoprostol active pharmaceutical ingredient (API) or Misoprostol finished formulations upon request. The Misoprostol suppliers may include Misoprostol API manufacturers, exporters, distributors and traders.
click here to find a list of Misoprostol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Misoprostol DMF (Drug Master File) is a document detailing the whole manufacturing process of Misoprostol active pharmaceutical ingredient (API) in detail. Different forms of Misoprostol DMFs exist exist since differing nations have different regulations, such as Misoprostol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Misoprostol DMF submitted to regulatory agencies in the US is known as a USDMF. Misoprostol USDMF includes data on Misoprostol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Misoprostol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Misoprostol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Misoprostol Drug Master File in Japan (Misoprostol JDMF) empowers Misoprostol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Misoprostol JDMF during the approval evaluation for pharmaceutical products. At the time of Misoprostol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Misoprostol suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Misoprostol Drug Master File in Korea (Misoprostol KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Misoprostol. The MFDS reviews the Misoprostol KDMF as part of the drug registration process and uses the information provided in the Misoprostol KDMF to evaluate the safety and efficacy of the drug.
After submitting a Misoprostol KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Misoprostol API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Misoprostol suppliers with KDMF on PharmaCompass.
A Misoprostol CEP of the European Pharmacopoeia monograph is often referred to as a Misoprostol Certificate of Suitability (COS). The purpose of a Misoprostol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Misoprostol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Misoprostol to their clients by showing that a Misoprostol CEP has been issued for it. The manufacturer submits a Misoprostol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Misoprostol CEP holder for the record. Additionally, the data presented in the Misoprostol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Misoprostol DMF.
A Misoprostol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Misoprostol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Misoprostol suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Misoprostol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Misoprostol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Misoprostol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Misoprostol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Misoprostol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Misoprostol suppliers with NDC on PharmaCompass.
Misoprostol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Misoprostol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Misoprostol GMP manufacturer or Misoprostol GMP API supplier for your needs.
A Misoprostol CoA (Certificate of Analysis) is a formal document that attests to Misoprostol's compliance with Misoprostol specifications and serves as a tool for batch-level quality control.
Misoprostol CoA mostly includes findings from lab analyses of a specific batch. For each Misoprostol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Misoprostol may be tested according to a variety of international standards, such as European Pharmacopoeia (Misoprostol EP), Misoprostol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Misoprostol USP).
