
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
Finished Drug Prices
NA
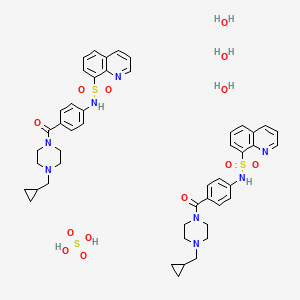

1. 8-quinolinesulfonamide, N-(4-((4-(cyclopropylmethyl)-1-piperazinyl)carbonyl)phenyl)-
2. Ag-348
3. Mitapivat
4. Mitapivat Hemisulfate Sesquihydrate
5. N-(4-((4-(cyclopropylmethyl)-1-piperazinyl)carbonyl)phenyl)-8-quinolinesulfonamide
6. N-(4-(4-(cyclopropylmethyl)piperazine-1-carbonyl)phenyl)quinoline-8-sulfonamide
7. Pyrukynd
1. Ag-348 Sulfate Hydrate
2. Mitapivat Sulfate [usan]
3. N4jta67v3o
4. Pyrukynd
5. Ag-348 Hemisulfate Sesquihydrate
6. Mitapivat Sulfate (usan)
7. 2151847-10-6
8. 8-quinolinesulfonamide, N-(4-((4-(cyclopropylmethyl)-1-piperazinyl)carbonyl)phenyl)-, Sulfate, Hydrate (2:1:3)
9. N-(4-(4-(cyclopropylmethyl)piperazine-1-carbonyl)phenyl)quinoline-8-sulfonamide Sulfate Hydrate (2:1:3)
10. Unii-n4jta67v3o
11. Chembl4297223
12. Mitapivat Hemisulfate Sesquihydrate
13. D11408
| Molecular Weight | 1053.2 g/mol |
|---|---|
| Molecular Formula | C48H60N8O13S3 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 12 |
| Exact Mass | 1052.34419752 g/mol |
| Monoisotopic Mass | 1052.34419752 g/mol |
| Topological Polar Surface Area | 268 Ų |
| Heavy Atom Count | 72 |
| Formal Charge | 0 |
| Complexity | 831 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 6 |
Enzyme Activators
Compounds or factors that act on a specific enzyme to increase its activity. (See all compounds classified as Enzyme Activators.)
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?