
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
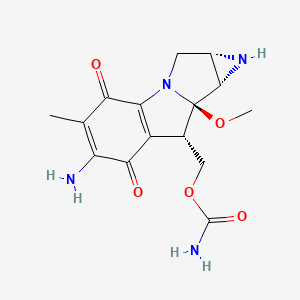

1. Ametycine
2. Mitocin C
3. Mitocin-c
4. Mitocinc
5. Mitomycin
6. Mitomycin-c
7. Mutamycin
8. Nsc 26980
9. Nsc-26980
10. Nsc26980
1. Mitomycin
2. 50-07-7
3. Ametycine
4. Mutamycin
5. Mitomycin-c
6. Mitocin-c
7. Mytozytrex
8. Ametycin
9. Mitomycinum
10. Mytomycin
11. Mitozytrex
12. Mitamycin
13. Mitosol
14. Mmc
15. Nsc-26980
16. Muamycin
17. 7-amino-9alpha-methoxymitosane
18. C15h18n4o5
19. Nsc 26980
20. Mitomycyna C
21. Nci-c04706
22. Mito-c
23. Nsc26980
24. Mit-c
25. Mitomycinum C
26. Mitocin C
27. Chebi:27504
28. Rcra Waste Number U010
29. Ugn-101
30. Ugn-102
31. 50sg953sk6
32. Mitomycyna C [polish]
33. 6-amino-1,1a,2,8,8a,8b-hexahydro-8-(hydroxymethyl)-8a-methoxy-5-methylazirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione Carbamate (ester)
34. Dsstox_cid_898
35. Dsstox_rid_75853
36. Dsstox_gsid_20898
37. Mitomycin (tn)
38. [(1as,8s,8ar,8bs)-6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl Carbamate
39. [(4s,6s,7r,8s)-11-amino-7-methoxy-12-methyl-10,13-dioxo-2,5-diazatetracyclo[7.4.0.0^{2,7}.0^{4,6}]trideca-1(9),11-dien-8-yl]methyl Carbamate
40. Azirino[2',3':3,4]pyrrolo[1,2-a]indole-4,7-dione, 6-amino-8-[[(aminocarbonyl)oxy]methyl]-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1as,8s,8ar,8bs)-
41. Cas-50-07-7
42. Muamycin (tn)
43. ((1as,8s,8ar,8bs)-6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazirino[2',3':3,4]pyrrolo[1,2-a]indol-8-yl)methyl Carbamate
44. [1as-(1a?,8?,8a?,8b?)]-6-amino-8-[[(aminocarbonyl)oxy]methyl]-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methylazirino[2',3':3,4]pyrrolo[1,2-a]indole-4,7-dione
45. Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-8-(((aminocarbonyl)oxy)methyl)-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1as,8s,8ar,8bs)-
46. Ccris 414
47. Hsdb 3239
48. Mitomycin (usp/inn)
49. Mls002702984
50. (amino-methoxy-methyl-dioxo-[?]yl)methyl Carbamate
51. Einecs 200-008-6
52. Rcra Waste No. U010
53. 7-amino-9.alpha.-methoxymitosane
54. Mitonco
55. Mitoplus
56. Mitoextra
57. Unii-50sg953sk6
58. Ai3-26199
59. Mitomycin [usan:usp:inn:ban]
60. Ncgc00095258-01
61. [(1as,8s,8ar,8bs)-6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazirino[2'',3'':3,4]pyrrolo[1,2-a]indol-8-yl]methyl Carbamate
62. [(1as,8s,8ar,8bs)-6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazirino[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl Carbamate
63. Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-1,1a,2,8,8a,8b-hexahydro-8-(hydroxymethyl)-8a-methoxy-5-methyl-, Carbamate (ester)
64. Jelmyto (tn)
65. Mfcd00078109
66. Jelmyto
67. Mitomycin C- Bio-x
68. Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-8-(((aminocarbonyl)oxy)methyl)-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1as-(1aalpha,8beta,8aalpha,8balpha))-
69. Mitomycin C From Streptomyces Caespitosus
70. Mitomycin [inn]
71. Mitomycin C (jp17)
72. Mitomycin [hsdb]
73. Mitomycin [usan]
74. Mitomycin [vandf]
75. Mitomycin C [mi]
76. Chembl105
77. Mitomycin [mart.]
78. Mitomycin C [jan]
79. Mitomycin [usp-rs]
80. Mitomycin [who-dd]
81. Mitomycin C [iarc]
82. Schembl3760
83. Cbiol_001927
84. Bspbio_001267
85. Kbiogr_000607
86. Kbioss_000607
87. Mitomycin C (4% In Nacl)
88. Mls001332654
89. Mitozytrex (tn) (supergene)
90. 50-07-7 (non-salt)
91. Ametycin Pound Notmitomycin C
92. Gtpl7089
93. Mitomycin [orange Book]
94. Dtxsid2020898
95. Mitomycin [ep Monograph]
96. Kbio2_000607
97. Kbio2_003175
98. Kbio2_005743
99. Kbio3_001073
100. Kbio3_001074
101. Ex-a501
102. Mitomycin [usp Monograph]
103. Bcpp000410
104. Bio1_000213
105. Bio1_000702
106. Bio1_001191
107. Bio2_000464
108. Bio2_000944
109. Hms1362o09
110. Hms1792o09
111. Hms1990o09
112. Hms2089f16
113. Hms3403o09
114. Amy10316
115. Azirino(2',3':3,4)pyrrolo(1,2-a)indole-4,7-dione, 6-amino-8-(((aminocarbonyl)oxy)methyl)-1,1a,2,8,8a,8b-hexahydro-8a-methoxy-5-methyl-, (1as-(1a.alpha.,8.beta.,8a.alpha.,8b.alpha.))-
116. Tox21_111493
117. Ac-918
118. Bdbm50428658
119. Gr-311
120. S8146
121. Zinc30726187
122. Akos015895703
123. Tox21_111493_1
124. Bcp9000285
125. Ccg-208564
126. Cs-0564
127. Db00305
128. Ks-5148
129. Idi1_002219
130. Smp1_000307
131. Mitomycin 100 Microg/ml In Acetonitrile
132. Ncgc00163468-02
133. Ncgc00163468-03
134. Ncgc00163468-05
135. Ncgc00163468-06
136. 1404-00-8
137. Bm164668
138. Hy-13316
139. Smr000058401
140. Bcp0726000181
141. M2320
142. C06681
143. D00208
144. D91590
145. Ab00918689-03
146. Ab00918689-04
147. 078m109
148. Mitomycin C, Antibiotic For Culture Media Use Only
149. Q-201410
150. Brd-k59670716-001-02-6
151. Brd-k59670716-001-06-7
152. Q19856779
153. Wln: T D3 B556 Bn Em Jv Mvttt&j Go1 H1ovz Kz L1
154. Mitomycin C From Streptomyces Caespitosus, >=970 Mug/mg (usp Xxiv)
155. Mitomycin C From Streptomyces Caespitosus, Powder, Bioreagent, Suitable For Cell Culture
156. Mitomycin C From Streptomyces Caespitosus, Powder, Contains Nacl As Solubilizer
157. Azirino[2',4]pyrrolo[1,2-a]indole-4,7-dione, 6-amino-1,1a,2,8,8a,8b-hexahydro-8-(hydroxymethyl)-8a- Methoxy-5-methyl-, Carbamate (ester)
158. Azirino[2',4]pyrrolo[1,2-a]indole-4,7-dione, 6-amino-8-[[(aminocarbonyl)oxy]methyl]-1,1a,2,8,8a,8b- Hexahydro-8a-methoxy-5-methyl-, [1ar-(1a.alpha.,8.beta.,8a.alpha.,8b.alpha.)]-
159. Mitomycin C From Streptomyces Caespitosus, >=98% (hplc), Potency: >=970 Mug Per Mg (usp Xxiv), Gamma-irradiated, Suitable For Cell Culture
| Molecular Weight | 334.33 g/mol |
|---|---|
| Molecular Formula | C15H18N4O5 |
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 334.12771969 g/mol |
| Monoisotopic Mass | 334.12771969 g/mol |
| Topological Polar Surface Area | 147 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 757 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Mitomycin |
| PubMed Health | Mitomycin |
| Drug Classes | Antibiotic, Antineoplastic Agent |
| Drug Label | Mitomycin (also known as mitomycin and/or mitomycin-C) is an antibiotic isolated from the broth of Streptomyces caespitosus which has been shown to have antitumor activity. The compound is heat stable, has a high melting point, and is freely soluble... |
| Active Ingredient | Mitomycin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 5mg/vial; 20mg/vial; 40mg/vial |
| Market Status | Prescription |
| Company | Bedford; Accord Hlthcare; Hikma Maple |
| 2 of 4 | |
|---|---|
| Drug Name | Mitosol |
| Active Ingredient | Mitomycin |
| Dosage Form | For solution |
| Route | Topical |
| Strength | 0.2mg/vial |
| Market Status | Prescription |
| Company | Mobius Therap |
| 3 of 4 | |
|---|---|
| Drug Name | Mitomycin |
| PubMed Health | Mitomycin |
| Drug Classes | Antibiotic, Antineoplastic Agent |
| Drug Label | Mitomycin (also known as mitomycin and/or mitomycin-C) is an antibiotic isolated from the broth of Streptomyces caespitosus which has been shown to have antitumor activity. The compound is heat stable, has a high melting point, and is freely soluble... |
| Active Ingredient | Mitomycin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 5mg/vial; 20mg/vial; 40mg/vial |
| Market Status | Prescription |
| Company | Bedford; Accord Hlthcare; Hikma Maple |
| 4 of 4 | |
|---|---|
| Drug Name | Mitosol |
| Active Ingredient | Mitomycin |
| Dosage Form | For solution |
| Route | Topical |
| Strength | 0.2mg/vial |
| Market Status | Prescription |
| Company | Mobius Therap |
Antibiotics, Antineoplastic; Nucleic Acid Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Mitomycin is useful for the palliative treatment of gastric adenocarcinoma, in conjunction with fluorouracil and doxorubicin. It has produced temporary beneficial effects in carcinomas of the cervix, colon, rectum, pancreas, breast, bladder, head and neck, and lung, and in melanoma. It has also shown activity against lymphomas and leukemia, particularly chronic granulocytic leukemia, but not in myeloma.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1247
Thirty patients with advanced colorectal adenocarcinoma were treated by chemotherapy with an alternating regimen consisting of 5-fluorouracil mitomycin C and 5-fluorouracil dacarbazine at 3 wk intervals. ... The toxicity of this regimen was essentially digestive with 30% of grade 3 or 4 nausea and vomiting. In spite of the reported active and synergistic action of drug association in colorectal carcinoma, this treatment schedule is not better than 5-fluorouracil alone. Gastrointestinal toxicity was incr.
PMID:2496369 Herait P et al; Oncology 46 (2): 88-90 (1989)
Forty-two patients with metastatic breast cancer refractory to first line therapies were treated with combination chemotherapy with mitomycin-C and vinblastine. ... The toxicity was acceptable with 20 episodes of moderate myelosuppression (58.8%) and 2 cases with congestive heart failure that responded to medical treatment.
PMID:2497417 Navarro M et al; Oncology 46 (3): 137-42 (1989)
For more Therapeutic Uses (Complete) data for MITOMYCIN C (19 total), please visit the HSDB record page.
Mitomycin is contraindicated in patients with pre-existing myelosupression & anemia.
Knoben, J.E. and P.O. Anderson (eds.) Handbook of Clinical Drug Data. 6th ed. Bethesda, MD: Drug Intelligence Publications, Inc. 1988., p. 417
Because normal defense mechanisms may be suppressed by mitomycin therapy, the patient's antibody response to the vaccine may be decreased. The interval between discontinuation of medications that cause immunosuppression and restoration of the patient's ability to respond to the vaccine depends on the intensity and type of immunosuppression-causing medication used, the underlying disease, and other factors; estimates vary from 3 months to 1 year.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2088
cBecause normal defense mechanisms may be suppressed by mitomycin therapy, concurrent use with a live virus vaccine may potentiate the replication of the vaccine virus, may increase the side/adverse effects of the vaccine virus, and/or may decrease the patient's antibody response to the vaccine; immunization of these patients should be undertaken only with extreme caution after careful review of the patient's hematologic status and only with the knowledge and consent of the physician managing the cytarabine therapy. The interval between discontinuation of medication that cause immunosuppression and restoration of the patient's ability to respond to the vaccine depends on the intensity and type of immunosuppression-causing medications used, the underlying disease, and other factors; estimates vary from 3 months to 1 year. Patients with leukemia in remission should not receive live virus vaccine until at least 3 months after their last chemotherapy. In addition, immunization with oral polio-virus vaccine should be postponed in persons in close contact with the patient, especially family members.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2088
Gonadal suppression, resulting in amenorrhea or azoospermia, may occur in patients taking antineoplastic therapy, especially with the alkylating agents. In general, these effects appear to be related to dose and length of therapy and may be irreversible. Prediction of the degree of testicular or ovarian function impairment is complicated by the common use of combinations of several antineoplastics, which makes it difficult to assess the effects of individual agents.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 2087
For more Drug Warnings (Complete) data for MITOMYCIN C (6 total), please visit the HSDB record page.
For treatment of malignant neoplasm of lip, oral cavity, pharynx, digestive organs, peritoneum, female breast, and urinary bladder. Also used as an adjunct to ab externo glaucoma surgery. Mitomycin is also indicated as a pyelocalyceal solution for the treatment of adults with low-grade upper tract urothelial cancer (LG-UTUC).
FDA Label
Mitomycin is one of the older chemotherapy drugs, which has been around and in use for decades. It is an antibiotic which has been shown to have antitumor activity. Mitomycin selectively inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed. Mitomycin has been shown in vitro to inhibit B cell, T cell, and macrophage proliferation and impair antigen presentation, as well as the secretion of interferon gamma, TNFa, and IL-2.
Alkylating Agents
Highly reactive chemicals that introduce alkyl radicals into biologically active molecules and thereby prevent their proper functioning. Many are used as antineoplastic agents, but most are very toxic, with carcinogenic, mutagenic, teratogenic, and immunosuppressant actions. They have also been used as components in poison gases. (See all compounds classified as Alkylating Agents.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
Cross-Linking Reagents
Reagents with two reactive groups, usually at opposite ends of the molecule, that are capable of reacting with and thereby forming bridges between side chains of amino acids in proteins; the locations of naturally reactive areas within proteins can thereby be identified; may also be used for other macromolecules, like glycoproteins, nucleic acids, or other. (See all compounds classified as Cross-Linking Reagents.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01D - Cytotoxic antibiotics and related substances
L01DC - Other cytotoxic antibiotics
L01DC03 - Mitomycin
Absorption
Erratic.
Route of Elimination
Approximately 10% of a dose of mitomycin is excreted unchanged in the urine.
FOLLOWING IV INJECTION OF 2 MG/KG BODY WT ... WISTAR RATS, 18% WAS RECOVERED UNCHANGED IN URINE WITHIN 24 HR AT ... 8 MG/KG ... 35% WAS RECOVERED IN URINE, BUT NONE IN FECES OR TISSUES.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 174 (1976)
THIRTY MIN AFTER IV INJECTION OF 8 MG/KG BODY WT TO MICE TRACES REMAINED IN BLOOD. IN GUINEA PIGS DRUG WAS CONCN IN KIDNEYS & NOT IN LIVER, SPLEEN OR BRAIN & WAS EXCRETED IN URINE.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 174 (1976)
Mitomycin is absorbed inconsistently from the gastrointestinal tract, and it is therefore administered intravenously. It disappears rapidly from the blood after injection. Peak concentrations in plasma are 0.4 ug/ml after doses of 20 mg/m sq ... The drug is widely distributed throughout the body but is not detected in the brain.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1247
In animals, highest mitomycin concentrations are found in the kidneys, followed by muscles, eyes, lung, intestines, and stomach. The drug is not detectable in the liver, spleen, or brain which rapidly inactivate mitomycin. Higher concentrations of the drug are generally present in cancer tissues than in normal tissues.
McEvoy, G.K. (ed.). AHFS Drug Information 90. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1990 (Plus Supplements 1990)., p. 537
For more Absorption, Distribution and Excretion (Complete) data for MITOMYCIN C (9 total), please visit the HSDB record page.
Primarily hepatic, some in various other tissues.
SUGGESTED ALKYLATING METABOLITES OF CARCINOGENS: MITOMYCIN C: REDUCTION PRODUCTS. /FROM TABLE/
Searle, C. E. (ed.). Chemical Carcinogens. ACS Monograph 173. Washington, DC: American Chemical Society, 1976., p. 95
Inactivation occurs by metabolism, but the products have not been identified. It is metabolized primarily in the liver, and less than 10% of the active drug is excreted in the urine or the bile.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1247
The drug is eliminated primarily by hepatic metabolism with about 20% hepatic extraction and 10-30% recovery of intact drug in the urine. Clearance is 0.3-0.4 l/hr/kg.
IATA. Dangerous Goods Regulations. 30th ed. Montreal, Canada: International Air Transport Association. Dangerous Goods Board, January 1, 1989., p. 416
Mitomycin disappears rapidly from the blood after intravenous injection. It is widely distributed but does not appear to cross the blood-brain barrier. Mitomycin is metabolized mainly in the liver; up to 10% of a dose is excreted unchanged in the urine.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 221
MITOMYCIN C WAS PREFERENTIALLY ACTIVATED & METABOLIZED BY SONICATED CELL PREPARATIONS. BIOACTIVATION OF MITOMYCIN TO ALKYLATING AGENT BY EMT6 & SARCOMA 180 CELL SONICATES REQUIRED HYPOXIC CONDITIONS & NADPH-GENERATING SYSTEM.
PMID:7388797 KENNEDY KA ET AL; CANCER RES 40 (7): 2356 (1980)
8-48 min
After doses of 20 mg/m sq ... Mitomycin is cleared from plasma with a half-time of approximately 1 hour.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1247
/Mitomycin/ has an alpha half-life of 5-10 min after IV injection and beta half-life of 46 min.
Knoben, J.E. and P.O. Anderson (eds.) Handbook of Clinical Drug Data. 6th ed. Bethesda, MD: Drug Intelligence Publications, Inc. 1988., p. 416
Mitomycin is activated in vivo to a bifunctional and trifunctional alkylating agent. Binding to DNA leads to cross-linking and inhibition of DNA synthesis and function. Mitomycin is cell cycle phase-nonspecific.
... REACTS WITH BACTERIAL DNA BUT NOT WITH ISOLATED DNA, UNLESS ... REDUCING SYSTEM IS ADDED. CROSS LINKING EFFICIENCY ... INCR IN ISOLATED BACTERIAL DNA CONTAINING INCR AMT OF CYTOSINE & GUANOSINE.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 175 (1976)
ITS REDUCED FORM CONTAINS INDOLE GROUP EMBODYING ALLYLIC CARBAMATE RESIDUE. ANTIBIOTIC IS CYTOTOXIC & CARCINOGENIC BUT IS INACTIVE AS CYTOTOXIC AGENT UNLESS REDUCED ... IT ACTS AS DIFUNCTIONAL AGENT IN CROSS LINKING DNA.
Searle, C. E. (ed.). Chemical Carcinogens. ACS Monograph 173. Washington, DC: American Chemical Society, 1976., p. 96
The drug inhibits DNA synthesis and cross-links DNA at the N6 position of adenine and at the O6 and N2 positions of guanine. In addition, single-strand breakage of DNA is caused by reduced mitomycin; this can be prevented by free radical scavengers. Its action is most prominent during the late G1 and early S phases of the cell cycle.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1247
In high concentrations ... /mitomycin/ may ... inhibit RNA and protein synthesis.
McEvoy, G.K. (ed.). AHFS Drug Information 90. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1990 (Plus Supplements 1990)., p. 537
For more Mechanism of Action (Complete) data for MITOMYCIN C (11 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
47
PharmaCompass offers a list of Mitomycin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mitomycin manufacturer or Mitomycin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mitomycin manufacturer or Mitomycin supplier.
PharmaCompass also assists you with knowing the Mitomycin API Price utilized in the formulation of products. Mitomycin API Price is not always fixed or binding as the Mitomycin Price is obtained through a variety of data sources. The Mitomycin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mitomycin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mitomycin, including repackagers and relabelers. The FDA regulates Mitomycin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mitomycin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Mitomycin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Mitomycin supplier is an individual or a company that provides Mitomycin active pharmaceutical ingredient (API) or Mitomycin finished formulations upon request. The Mitomycin suppliers may include Mitomycin API manufacturers, exporters, distributors and traders.
click here to find a list of Mitomycin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Mitomycin DMF (Drug Master File) is a document detailing the whole manufacturing process of Mitomycin active pharmaceutical ingredient (API) in detail. Different forms of Mitomycin DMFs exist exist since differing nations have different regulations, such as Mitomycin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Mitomycin DMF submitted to regulatory agencies in the US is known as a USDMF. Mitomycin USDMF includes data on Mitomycin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Mitomycin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Mitomycin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Mitomycin Drug Master File in Japan (Mitomycin JDMF) empowers Mitomycin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Mitomycin JDMF during the approval evaluation for pharmaceutical products. At the time of Mitomycin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Mitomycin suppliers with JDMF on PharmaCompass.
A Mitomycin CEP of the European Pharmacopoeia monograph is often referred to as a Mitomycin Certificate of Suitability (COS). The purpose of a Mitomycin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Mitomycin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Mitomycin to their clients by showing that a Mitomycin CEP has been issued for it. The manufacturer submits a Mitomycin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Mitomycin CEP holder for the record. Additionally, the data presented in the Mitomycin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Mitomycin DMF.
A Mitomycin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Mitomycin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Mitomycin suppliers with CEP (COS) on PharmaCompass.
A Mitomycin written confirmation (Mitomycin WC) is an official document issued by a regulatory agency to a Mitomycin manufacturer, verifying that the manufacturing facility of a Mitomycin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Mitomycin APIs or Mitomycin finished pharmaceutical products to another nation, regulatory agencies frequently require a Mitomycin WC (written confirmation) as part of the regulatory process.
click here to find a list of Mitomycin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Mitomycin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Mitomycin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Mitomycin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Mitomycin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Mitomycin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Mitomycin suppliers with NDC on PharmaCompass.
Mitomycin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mitomycin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mitomycin GMP manufacturer or Mitomycin GMP API supplier for your needs.
A Mitomycin CoA (Certificate of Analysis) is a formal document that attests to Mitomycin's compliance with Mitomycin specifications and serves as a tool for batch-level quality control.
Mitomycin CoA mostly includes findings from lab analyses of a specific batch. For each Mitomycin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mitomycin may be tested according to a variety of international standards, such as European Pharmacopoeia (Mitomycin EP), Mitomycin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mitomycin USP).
