
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Chloditan
2. Chlodithane
3. Khloditan
4. Lysodren
5. Mytotan
6. O,p-ddd
7. Ortho,para Ddd
8. Ortho,para-ddd
1. 53-19-0
2. Mitotan
3. Lysodren
4. O,p'-ddd
5. Chlodithane
6. Chloditan
7. Khlodithan
8. Chlodithan
9. 2,4'-ddd
10. 1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane
11. O,p'-tde
12. 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]benzene
13. Mitotano
14. Opeprim
15. Nci-c04933
16. 2,4'-dichlorodiphenyldichloroethane
17. 1-chloro-2-(2,2-dichloro-1-(4-chlorophenyl)ethyl)benzene
18. O,p'-dichlorodiphenyldichloroethane
19. Cb 313
20. 2,4'-dichlorophenyldichlorethane
21. Benzene, 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]-
22. Cb-313
23. Nsc 38721
24. Nsc-38721
25. 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane
26. 2-(2-chlorophenyl)-2-(4-chlorophenyl)-1,1-dichloroethane
27. 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1-dichloroethane
28. 1,1-dichloro-2,2-bis(2,4'-dichlorophenyl)ethane
29. O,p'-ddd,o P'-tde
30. Nsc38721
31. 1,1-dichloro-2-(p-chlorophenyl)-2-(o-chlorophenyl)ethane
32. 2,2-bis(2-chlorophenyl-4-chlorophenyl)-1,1-dichloroethane
33. Benzene, 1-chloro-2-(2,2-dichloro-1-(4-chlorophenyl)ethyl)-
34. 78e4j5ib5j
35. 2,4'-ddd;o,p'-ddd
36. Ethane, 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)-
37. Ethane, 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1-dichloro-
38. 2,4 Inverted Exclamation Marka-ddd
39. Mitotanum [inn-latin]
40. O,p-tde
41. Ncgc00015226-07
42. 1-chloro-4-[2,2-dichloro-1-(2-chlorophenyl)ethyl]benzene
43. 2,4'-ddd 10 Microg/ml In Cyclohexane
44. Mitotanum
45. 2,4'-ddd 100 Microg/ml In Cyclohexane
46. Dsstox_cid_372
47. Mitotano [inn-spanish]
48. Dsstox_rid_75548
49. Dsstox_gsid_20372
50. 2,4'-(2,2-dichloroethane-1,1-diyl)bis(chlorobenzene)
51. Cb313
52. Ddd, O,p'-
53. Cas-53-19-0
54. Lysodren (tn)
55. Smr000326696
56. Ccris 4397
57. Hsdb 3240
58. Sr-01000075751
59. Einecs 200-166-6
60. (o,p)-ddd
61. (2,4'-dichlorodiphenyl)dichloroethane
62. Mitotane (jan/usp/inn)
63. Brn 2056007
64. Unii-78e4j5ib5j
65. Ai3-07575
66. Prestwick_75
67. Ethane,1-dichloro-
68. Mfcd00000850
69. Mitotane (lysodren)
70. Mitotane [usan:usp:inn:ban:jan]
71. Spectrum_001959
72. Mitotane [hsdb]
73. Mitotane [usan]
74. Ddd-o,p'
75. Mitotane [inn]
76. Mitotane [jan]
77. Mitotane [mi]
78. Mitotane [vandf]
79. (+-)-1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane
80. Spectrum2_000916
81. Spectrum3_000869
82. Spectrum4_000709
83. Spectrum5_002060
84. Mitotan-[ring-13c6]
85. Mitotane [mart.]
86. Mitotane [usp-rs]
87. Mitotane [who-dd]
88. C 3010
89. (o,p')-ddd
90. Schembl4040
91. Chembl1670
92. Mitotane [ema Epar]
93. Lopac0_000251
94. Wln: Gygyr Bg&r Dg
95. Bspbio_002517
96. Kbiogr_001237
97. Kbioss_002513
98. Spectrum330082
99. Mls001335899
100. Mls001335900
101. Mls002152914
102. Mls002153233
103. Divk1c_000703
104. Spbio_000792
105. Mitotane [orange Book]
106. Chebi:6954
107. Gtpl6957
108. Mitotane [usp Impurity]
109. Mitotane, >=98% (hplc)
110. Dtxsid9020372
111. Mitotane [usp Monograph]
112. 2,4'-ddd, Analytical Standard
113. Hms502d05
114. Kbio1_000703
115. Kbio2_002505
116. Kbio2_005073
117. Kbio2_007641
118. Kbio3_002017
119. Ninds_000703
120. Hms1923k19
121. Hms2091e07
122. Hms2232c16
123. Hms3260d04
124. Hms3369h10
125. Hms3655g06
126. Hms3715h07
127. Hms3869f13
128. Pharmakon1600-00330082
129. Bcp11663
130. Tox21_110103
131. Tox21_302804
132. Tox21_500251
133. Ccg-40014
134. Nsc755849
135. S1732
136. 1,2-bis(2,4'-dichlorophenyl)ethane
137. Akos006028802
138. Benzene, 1-chloro-2-(2,2-dichloro-1-(4-chlorophenyl)ethyl)-, (+-)-
139. Tox21_110103_1
140. Cs-1500
141. Db00648
142. Lp00251
143. Nsc-755849
144. Sdccgsbi-0050239.p006
145. Idi1_000703
146. 2,4'-ddd 100 Microg/ml In Methanol
147. Ncgc00015226-02
148. Ncgc00015226-03
149. Ncgc00015226-04
150. Ncgc00015226-05
151. Ncgc00015226-06
152. Ncgc00015226-08
153. Ncgc00015226-09
154. Ncgc00015226-10
155. Ncgc00015226-11
156. Ncgc00015226-12
157. Ncgc00091374-01
158. Ncgc00091374-02
159. Ncgc00091374-03
160. Ncgc00091374-04
161. Ncgc00091374-05
162. Ncgc00091374-06
163. Ncgc00256452-01
164. Ncgc00260936-01
165. As-11690
166. Hy-13690
167. Nci60_003688
168. 2,4'-ddd 1000 Microg/ml In N-hexane
169. Sbi-0050239.p004
170. Eu-0100251
171. Ft-0605518
172. M3304
173. Sw199619-3
174. En300-37268
175. 2,4 Inverted Exclamation Marka-ddd;o,p'-ddd
176. 2,4'-ddd, Pestanal(r), Analytical Standard
177. C75491
178. D00420
179. Ab00052337-09
180. Ab00052337_10
181. 000m850
182. Q417465
183. Sr-01000075751-1
184. Sr-01000075751-3
185. Sr-01000075751-6
186. Z425389592
187. 1-(o-chlorophenyl)-1-(p-chlorophenyl)-2,2-dichloroethane
188. Ethane,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)-
189. 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]benzene #
190. Mitotane, United States Pharmacopeia (usp) Reference Standard
191. (+/-)-1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane
192. 1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane, Analytical Standard
193. Benzene, 1-chloro-2-(2,2-dichloro-1-(4-chlorophenyl)ethyl)-, (+/-)-
194. 1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane For Diagnostic Uses (cancer Investigation), >=98% (hplc)
195. 1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane, For Diagnostic Uses (cancer Investigation), >=98% (hplc)
| Molecular Weight | 320.0 g/mol |
|---|---|
| Molecular Formula | C14H10Cl4 |
| XLogP3 | 6.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 3 |
| Exact Mass | 319.950711 g/mol |
| Monoisotopic Mass | 317.953661 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 248 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Lysodren |
| PubMed Health | Mitotane (By mouth) |
| Drug Classes | Adrenocortical Suppressant, Steroid Synthesis Inhibitor |
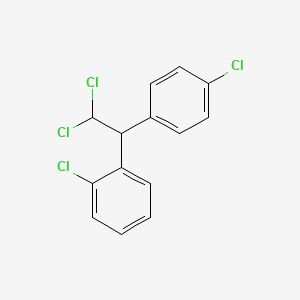
| Drug Label | LYSODREN (mitotane tablets, USP) is an oral chemotherapeutic agent. It is best known by its trivial name, o,p-DDD, and is chemically, 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane. The chemical structure is shown below:LYSODREN is a... |
| Active Ingredient | Mitotane |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 2 of 2 | |
|---|---|
| Drug Name | Lysodren |
| PubMed Health | Mitotane (By mouth) |
| Drug Classes | Adrenocortical Suppressant, Steroid Synthesis Inhibitor |
| Drug Label | LYSODREN (mitotane tablets, USP) is an oral chemotherapeutic agent. It is best known by its trivial name, o,p-DDD, and is chemically, 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane. The chemical structure is shown below:LYSODREN is a... |
| Active Ingredient | Mitotane |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
Antineoplastic Agents, Hormonal
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Mitotane is indicated in the treatment of inoperable adrenal cortical carcinoma of both functional and nonfunctional types. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for LYSODREN (mitotane) tablets (March 2009). Available from, as of June 22, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=9478
Mitotane is also used in the treatment of Cushing's syndrome. /Not included in US or Canadian product labeling/
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1937
The effect of mitotane therapy in treatment of adrenocortical carcinoma was studied in 88 patients (mean age 46 yr); 80 of these patients underwent surgery and 59 also received oral mitotane capsule, at a mean initial dose of 10 g/day and a mean maintenance dose of 7 g/day for a mean duration of 10.5 months. The median disease free interval after surgery was 12.1 months. Tumor dissemination occurred in 82% of the patients, most commonly to the lung, liver and adjacent organs. The median survival time was 14.5 months, and the 5 yr survival was 22%. Age over 40 yr and the presence of metastases at the time of diagnosis were the only factors recognized as indicating a poor prognosis. Mitotane controlled hormonal secretion in 75% of the patients. Eight mitotane treated patients had partial tumor regression, but the drug did not have a significant effect on survival. It was concluded that adrenocortical carcinoma carries a poor prognosis; mitotane therapy may offer transient benefits, particularly in controlling endocrine symptoms.
Luton JP et al; N Eng J Med 322 (26): 1195-1201 (1990)
/BOXED WARNING/ WARNINGS: Lysodren (mitotane tablets, USP) should be administered under the supervision of a qualified physician experienced in the uses of cancer chemotherapeutic agents. Lysodren should be temporarily discontinued immediately following shock or severe trauma since adrenal suppression is its prime action. Exogenous steroids should be administered in such circumstances, since the depressed adrenal may not immediately start to secrete steroids.
US Natl Inst Health; DailyMed. Current Medication Information for LYSODREN (mitotane) tablets (Updated: November 2013). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e9fba7d4-a0ec-4bfa-9b5b-ec97a9710fd3
... Seventeen consecutive patients who were treated with mitotane after radical resection of adrenocortical cancer (ACC) from 1999 to 2005 underwent physical examination, routine laboratory evaluation, monitoring of mitotane concentrations, and a hormonal work-up at baseline and every 3 months till ACC relapse or study end (December 2007). Mitotane toxicity was graded using NCI CTCAE criteria. All biochemical measurements were performed at our center and plasma mitotane was measured by an in-house HPLC assay. All the patients reached mitotane concentrations >14 mg/L and none of them discontinued definitively mitotane for toxicity; 14 patients maintained consistently elevated mitotane concentrations despite tapering of the drug. Side effects occurred in all patients but were manageable with palliative treatment and adjustment of hormone replacement therapy. Mitotane affected adrenal steroidogenesis with a more remarkable inhibition of cortisol and DHEAS than aldosterone. Mitotane induced either perturbation of thyroid function mimicking central hypothyroidism or, in male patients, inhibition of testosterone secretion. The discrepancy between salivary and serum cortisol, as well as between total and free testosterone, is due to the mitotane-induced increase in hormone-binding proteins which complicates interpretation of hormone measurements. ...
PMID:18824557 Daffara F et al; Endocr Relat Cancer 15 (4): 1043-53 (2008).
/Investigators/ prospectively studied 7 patients with adrenocortical cancer on mitotane therapy. Before and 1 and 2 or more weeks after starting mitotane we determined the platelet counts, bleeding times and global coagulation parameters. All patients had a normal bleeding time before treatment. In 6 cases the bleeding time became prolonged (245-555 s). Four patients exhibited platelet aggregation responses compatible with an aspirin-like defect. It is concluded that mitotane may cause a clinically relevant defect of platelet function.
PMID:1828976 Haak HR et al; Eur J Cancer 27 (5): 638-41(1991).
Gastrointestinal disturbances (anorexia, nausea, vomiting, and diarrhea) occur in 80% of patients and are usually dose limiting. About 40% of patients experience central nervous system side effects (lethargy and somnolence, 25%; dizziness or vertigo, 15%). About 15% of patients develop dermatitis.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 2081
For more Drug Warnings (Complete) data for MITOTANE (28 total), please visit the HSDB record page.
For treatment of inoperable adrenocortical tumours; Cushing's syndrome
Symptomatic treatment of advanced (unresectable, metastatic or relapsed) adrenal cortical carcinoma. The effect of Lysodren on non-functional adrenal cortical carcinoma is not established.
Mitotane is an oral chemotherapeutic agent indicated in the treatment of inoperable adrenal cortical carcinoma of both functional and nonfunctional types. Mitotane can best be described as an adrenal cytotoxic agent, although it can cause adrenal inhibition, apparently without cellular destruction. The administration of Mitotane alters the extra-adrenal metabolism of cortisol in man; leading to a reduction in measurable 17-hydroxy corticosteroids, even though plasma levels of corticosteroids do not fall. The drug apparently causes increased formation of 6-B-hydroxyl cortisol.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
L01XX23
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX23 - Mitotane
Absorption
About 40% oral Lysodren is absorbed
Route of Elimination
A variable amount of metabolite (1%-17%) is excreted in the bile and the balance is apparently stored in the tissues.
Clinical studies indicate that approximately 40% of mitotane is absorbed after oral administration. After daily doses of 5 to 15 g, concentrations of 10 to 90 ug/mL of unchanged drug and 30 to 50 ug/mL of a metabolite are present in the blood. After discontinuation of therapy, plasma concentrations of mitotane are still measurable for 6 to 9 weeks. Although the drug is found in all tissues, fat is the primary site of storage.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1253
Peak plasma mitotane concentrations occur 3-5 hours after a single oral dose of the drug and distribution of the drug between plasma and tissues is complete within 12 hours. In one study in patients with adrenocortical carcinoma who were receiving an oral dosage of 5-15 g of mitotane daily, serum mitotane concentrations were 7-90 ug/mL and serum concentrations of mitotane metabolites were 29-54 ug/mL. Serum concentrations of mitotane and its metabolites appear to plateau after about 8 weeks of continuous mitotane therapy and generally have not appeared to correlate with therapeutic or toxic effects of the drug; however, some data suggest that tumor regression in patients with adrenocortical carcinoma is associated with serum mitotane concentrations greater than 14 ug/mL and that adverse CNS effects are associated with serum concentrations greater than 20 ug/mL.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
Mitotane and its metabolites are distributed to essentially all body tissues with fat being the primary storage site; there is no selective accumulation in the adrenals. Following discontinuance of mitotane therapy, persistent plasma concentrations of mitotane and its metabolites are probably caused by their slow release from fat and other tissues. Although unchanged mitotane has not been detected in CSF, small amounts of one mitotane metabolite have been detected in CSF.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
It is not known if mitotane or its metabolites cross the placenta or distribute into milk.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
For more Absorption, Distribution and Excretion (Complete) data for MITOTANE (11 total), please visit the HSDB record page.
Hepatic and renal
In rabbits and in humans, ortho,para'-dichlorodiphenylacetic acid has been identified as major urinary metabolite.../of mitotane/.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 324
With oral dosing of mitotane, metabolites o,p'-dichlorodiphenylacetic acid and its mono- and dihydroxylated derivatives appeared in urine as well as in stools. An unsaturated metabolite, o,p'-DDE was observed in plasma & tissues of man.
TOUITOU Y ET AL; ANN ENDOCRINOL (PARIS) 38 13 (1977)
Urine samples were obtained from four patients with Cushing syndrome who had been treated with o,p'-DDD. Methyl-thio containing metabolites and other metabolites of o,p'-DDD were detected in the urine samples by gas chromatographic mass spectrometry. These methyl-thio containing metabolites showed smaller peaks in the gas chromatogram than other peaks derived from other metabolites of o,p'-DDD. Although the biological significance of the pathway forming these methyl-thio containing metabolites is not known at the present /time/. ...
PMID:2724646 Inouye M et al; Jpn J Med 28 (1): 41-4 (1989)
Mitotane is metabolized in the liver and other tissues principally to o,p'-dichlorodiphenyl-ethene and -acetate derivatives; small amounts of these derivatives apparently undergo aromatic hydroxylation and glycine conjugation.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
For more Metabolism/Metabolites (Complete) data for MITOTANE (6 total), please visit the HSDB record page.
18-159 days
Mitotane reportedly has a plasma elimination half-life of 18-159 days.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
Its biochemical mechanism of action is unknown, although data are available to suggest that the drug modifies the peripheral metabolism of steroids as well as directly suppressing the adrenal cortex.
The exact mechanisms of action of mitotane have not been clearly established. Although mitotane is an adrenocortical cytotoxic agent, the drug can also apparently inhibit adrenocortical function without causing cellular destruction. Mitotane appears to selectively inhibit adrenocortical function as well as functional and nonfunctional adrenocortical neoplasms by a direct cytotoxic effect; this effect may be mediated through covalent bonding of mitotane metabolites to mitochondrial proteins. The drug causes focal degeneration in the zona fasciculata and reticularis of the adrenal cortex with resultant atrophy. The drug usually causes only minimal degeneration in the zona glomerulosa (site of aldosterone biosynthesis); however, the zona glomerulosa may be damaged with prolonged mitotane therapy. Mitotane also reportedly inhibits the growth of human renal carcinoma cells, astrocytoma cells, and fibroblasts in vitro.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
Mitotane inhibits production of corticosteroids and alters extra-adrenal metabolism of endogenous and exogenous steroids. Mitotane inhibits the normal 11-beta-hydroxylation of 11-deoxycortisol (compound S) and 11-deoxycorticosterone (DOC) in the adrenal cortex, thus blocking conversion of compound S to cortisol (hydrocortisone) and DOC to corticosterone. The drug can also inhibit 18-hydroxylase activity in the adrenal cortex and thus decrease the production of aldosterone by blocking the conversion of corticosterone to 18-hydroxycorticosterone (the immediate precursor of aldosterone). Mitotane decreases cortisol secretion rate; plasma cortisol concentration; urinary excretion of free cortisol, 17-hydroxycorticosteroids (17-OHCS), 17-ketosteroids (17-KS), and 17-ketogenic steroids; and adrenocortical response to stimulation by corticotropin (ACTH). A feedback increase in plasma corticotropin concentration is generally observed in patients receiving mitotane; however, a lack of feedback increase or a decrease in plasma corticotropin concentration has been observed in some patients. Therefore, it has been suggested that the drug may have a partial suppressive effect on pituitary corticotropin-secreting cells. Mitotane apparently increases the extra-adrenal metabolism of cortisol to 6-beta-hydroxycortisol which results in decreased urinary excretion of measurable 17-OHCS; this occurs even in the presence of unchanged cortisol secretion rate or plasma cortisol concentration. Although urinary excretion of aldosterone metabolites may decrease, serum aldosterone concentrations may remain in the normal range. Therefore, it has been suggested that mitotane may also alter the extra-adrenal metabolism of aldosterone. Mitotane decreases the extra-adrenal conversion of androgens to androsterone and etiocholanolone; this results in decreased urinary excretion of 17-KS. The drug also inhibits the extra-adrenal conversion of 3-beta-hydroxysteroids to 3-alpha-hydroxypregnane derivatives.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
Mitotane has uricosuric activity and thus decreases serum uric acid concentrations; the exact mechanism of the increase in renal uric acid clearance has not been established.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1178
Mitotane can best be described as an adrenal cytotoxic agent, although it can cause adrenal inhibition, apparently without cellular destruction. Its biochemical mechanism of action is unknown. Data are available to suggest that the drug modifies the peripheral metabolism of steroids as well as directly suppressing the adrenal cortex. The administration of mitotane alters the extra-adrenal metabolism of cortisol in man; leading to a reduction in measurable 17-hydroxy corticosteroids, even though plasma levels of corticosteroids do not fall. The drug apparently causes increased formation of 6-beta-hydroxycortisol.
US Natl Inst Health; DailyMed. Current Medication Information for LYSODREN (mitotane) tablets (March 2009). Available from, as of June 22, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=9478
For more Mechanism of Action (Complete) data for MITOTANE (7 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE
45
PharmaCompass offers a list of Mitotane API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mitotane manufacturer or Mitotane supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mitotane manufacturer or Mitotane supplier.
PharmaCompass also assists you with knowing the Mitotane API Price utilized in the formulation of products. Mitotane API Price is not always fixed or binding as the Mitotane Price is obtained through a variety of data sources. The Mitotane Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mitotane manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mitotane, including repackagers and relabelers. The FDA regulates Mitotane manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mitotane API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Mitotane manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Mitotane supplier is an individual or a company that provides Mitotane active pharmaceutical ingredient (API) or Mitotane finished formulations upon request. The Mitotane suppliers may include Mitotane API manufacturers, exporters, distributors and traders.
click here to find a list of Mitotane suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Mitotane DMF (Drug Master File) is a document detailing the whole manufacturing process of Mitotane active pharmaceutical ingredient (API) in detail. Different forms of Mitotane DMFs exist exist since differing nations have different regulations, such as Mitotane USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Mitotane DMF submitted to regulatory agencies in the US is known as a USDMF. Mitotane USDMF includes data on Mitotane's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Mitotane USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Mitotane suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Mitotane Drug Master File in Japan (Mitotane JDMF) empowers Mitotane API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Mitotane JDMF during the approval evaluation for pharmaceutical products. At the time of Mitotane JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Mitotane suppliers with JDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Mitotane as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Mitotane API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Mitotane as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Mitotane and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Mitotane NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Mitotane suppliers with NDC on PharmaCompass.
Mitotane Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mitotane GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mitotane GMP manufacturer or Mitotane GMP API supplier for your needs.
A Mitotane CoA (Certificate of Analysis) is a formal document that attests to Mitotane's compliance with Mitotane specifications and serves as a tool for batch-level quality control.
Mitotane CoA mostly includes findings from lab analyses of a specific batch. For each Mitotane CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mitotane may be tested according to a variety of international standards, such as European Pharmacopoeia (Mitotane EP), Mitotane JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mitotane USP).

