

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada

0
Australia

0
Listed Dossiers
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
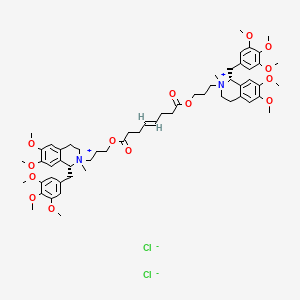

1. Bw B 1090u
2. Bw B1090u
3. Bw-b 1090u
4. Bw-b-1090u
5. Bw-b1090u
6. Bwb 1090u
7. Bwb1090u
8. Mivacron
9. Mivacurium
1. Mivacron
2. 106861-44-3
3. Mivacurium Chloride (mixture Of Isomers)
4. Bw-b 1090u
5. Mivacurium (dichloride)
6. Bis[3-[(1r)-6,7-dimethoxy-2-methyl-1-[(3,4,5-trimethoxyphenyl)methyl]-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propyl] (e)-oct-4-enedioate;dichloride
7. Bwb109ou
8. Bw B109ou Dichloride
9. Mivacurii Chloridum
10. Mivacurii Chloridum [latin]
11. Cloruro De Mivacurio
12. Cloruro De Mivacurio [spanish]
13. Chlorure De Mivacurium
14. Chlorure De Mivacurium [french]
15. Ncgc00167469-01
16. Mivacurium Chloride [usan:inn:ban]
17. Unii-600zg213c3
18. Bw-b1090u
19. Mivacron (tn)
20. Mivacron In Dextrose 5% In Plastic Container
21. Bw B1090u Dichloride
22. Chembl984
23. Dsstox_cid_26649
24. Dsstox_rid_81794
25. Dsstox_gsid_46649
26. Schembl41232
27. (r)-1,2,3,4-tetrahydro-2-(3-hydroxypropyl)-6,7-dimethoxy-2-methyl-1-(3,4,5-trimethoxybenzyl)isoquinolinium Chloride, (e)-4-octenedioate (2:1)
28. Bw-b109ou Dichloride
29. Bw-1090u Dichloride
30. Chebi:6959
31. Dtxsid5046649
32. Schembl10666657
33. Hy-b1700a
34. Mivacurium Chloride (usan/inn)
35. Hms3886m16
36. Tox21_112473
37. Akos025311198
38. 600zg213c3
39. Isoquinolinium, 2,2'-((1,8-dioxo-4-octene-1,8-diyl)bis(oxy-3,1-propanediyl))bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-((3,4,5-trimethoxyphenyl)methyl)-, Dichloride, (r-(r*,r*-(e)))-
40. Cas-106861-44-3
41. Cs-0013697
42. D00763
43. Q27263131
| Molecular Weight | 1100.2 g/mol |
|---|---|
| Molecular Formula | C58H80Cl2N2O14 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 30 |
| Exact Mass | 1098.4986606 g/mol |
| Monoisotopic Mass | 1098.4986606 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 76 |
| Formal Charge | 0 |
| Complexity | 1550 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Neuromuscular Nondepolarizing Agents
Drugs that interrupt transmission at the skeletal neuromuscular junction without causing depolarization of the motor end plate. They prevent acetylcholine from triggering muscle contraction and are used as muscle relaxants during electroshock treatments, in convulsive states, and as anesthesia adjuvants. (See all compounds classified as Neuromuscular Nondepolarizing Agents.)
M - Musculo-skeletal system
M03 - Muscle relaxants
M03A - Muscle relaxants, peripherally acting agents
M03AC - Other quaternary ammonium compounds
M03AC10 - Mivacurium chloride

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
