
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
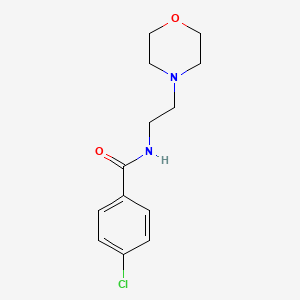

1. Apo Moclobemide
2. Apo-moclobemide
3. Arima
4. Aurorex
5. Aurorix
6. Azu, Moclobemid
7. Chem Mart Moclobemide
8. Dbl Moclobemide
9. Deprenorm
10. Feraken
11. Genrx Moclobemide
12. Healthsense Moclobemide
13. Manerix
14. Moclamine
15. Moclix
16. Moclobamide
17. Moclobemid 1a Pharma
18. Moclobemid Azu
19. Moclobemid Puren
20. Moclobemid Ratiopharm
21. Moclobemid Stada
22. Moclobemid Von Ct
23. Moclobemid-1a Pharma
24. Moclobemid-puren
25. Moclobemid-ratiopharm
26. Moclobemid1a Pharma
27. Moclobemide, Chem Mart
28. Moclobemide, Dbl
29. Moclobemide, Genrx
30. Moclobemide, Healthsense
31. Moclobeta
32. Moclodura
33. Moclonorm
34. Novo Moclobemide
35. Novo-moclobemide
36. Novomoclobemide
37. Nu Moclobemide
38. Nu-moclobemide
39. Numoclobemide
40. Pms Moclobemide
41. Pms-moclobemide
42. Rimoc
43. Ro 11 1163
44. Ro 11-1163
45. Ro-11-1163
46. Stada, Moclobemid
47. Terry White Chemists Moclobemide
48. Von Ct, Moclobemid
1. 71320-77-9
2. Aurorix
3. Moclobemid
4. Manerix
5. Moclamine
6. 4-chloro-n-(2-morpholinoethyl)benzamide
7. Moclaime
8. Moclobemidum
9. Moclamide
10. Moclobemida
11. P-chloro-n-(2-morpholinoethyl)benzamide
12. Moclobemidum [inn-latin]
13. Moclobemida [inn-spanish]
14. 4-chloro-n-[2-(morpholin-4-yl)ethyl]benzamide
15. 4-chlor-n-(2-morpholinoethyl)benzamid
16. Gnf-pf-695
17. 4-chloro-n-(2-morpholin-4-ylethyl)benzamide
18. 4-chloro-n-(2-(4-morpholinyl)ethyl)benzamide
19. 4-chloro-n-[2-(4-morpholinyl)ethyl]benzamide
20. Ro 11-1163
21. 4-chloro-n-(2-morpholin-4-yl-ethyl)-benzamide
22. Moclobemide (ro 111163)
23. Ro 11-1163/000
24. Ro111163
25. Benzamide, 4-chloro-n-(2-(4-morpholinyl)ethyl)-
26. Benzamide, 4-chloro-n-[2-(4-morpholinyl)ethyl]-
27. Pj0y7azb63
28. Chembl86304
29. Mls000070549
30. Chebi:83531
31. Ncgc00027930-02
32. Ncgc00027930-04
33. Smr000012114
34. Ro-111163000
35. Ro-11-1163/000
36. Dsstox_cid_20554
37. Dsstox_rid_79506
38. Dsstox_gsid_40554
39. Moclobemide [usan:ban:inn]
40. Moclobemide [usan:inn:ban]
41. Auromid
42. Aurorix (tn)
43. Cas-71320-77-9
44. Ro-11-1163
45. Hsdb 7180
46. Sr-01000357772
47. Cbmicro_048319
48. Moclobemide (usan/inn)
49. Unii-pj0y7azb63
50. Brn 0530974
51. Moclobemide- Bio-x
52. Mfcd00865388
53. Moclamine (salt/mix)
54. Opera_id_225
55. Moclobemide [mi]
56. Moclobemide [inn]
57. Moclobemide [hsdb]
58. Moclobemide [usan]
59. Moclobemide [mart.]
60. Oprea1_256739
61. Oprea1_270122
62. Schembl49708
63. Moclobemide [who-dd]
64. Mls000759438
65. Mls001240195
66. Mls001424077
67. Gtpl7428
68. Benzamide,4-chloro-n-[2-(4-morpholinyl)ethyl]-
69. Dtxsid9040554
70. Bdbm15613
71. Hms2051a16
72. Hms2096g07
73. Hms2232b20
74. Hms3262f09
75. Hms3371a01
76. Hms3393a16
77. Hms3657k05
78. Hms3713g07
79. Hms3885a15
80. Amy32534
81. Bcp15783
82. Hy-b0534
83. Moclobemide 1.0 Mg/ml In Methanol
84. Tox21_110971
85. Tox21_113614
86. Tox21_500824
87. S3212
88. Stk222240
89. Zinc19606670
90. Akos003270184
91. Moclobemide, >=98% (hplc), Solid
92. Tox21_110971_1
93. Ccg-100879
94. Db01171
95. Lp00824
96. Nc00129
97. Sdccgsbi-0048213.p003
98. Ncgc00027930-03
99. Ncgc00027930-05
100. Ncgc00027930-07
101. Ncgc00027930-16
102. Ncgc00261509-01
103. Ac-12467
104. Ac-35244
105. Bm164599
106. P-chloro-n-(2-morpholinoethyl)-benzamide
107. Bim-0048213.p001
108. Ft-0618228
109. M2733
110. Sw197509-3
111. D02561
112. Ab00400932_12
113. 320m779
114. A837152
115. Q421934
116. 4-chloro-n-(2-morpholinoethyl)benzamide;moclobemide
117. Sr-01000357772-1
118. Sr-01000357772-4
119. Z32409934
120. Ro11-1163; Ro 11-1163; Ro111163; Ro-111163; Ro 111163
121. 108375-13-9
| Molecular Weight | 268.74 g/mol |
|---|---|
| Molecular Formula | C13H17ClN2O2 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 268.0978555 g/mol |
| Monoisotopic Mass | 268.0978555 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 262 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Moclobemide is indicated for the relief of symptoms of depressive illness. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1931
Antidepressants appear to be useful in the treatment of pain disorders.
PMID:14608245 Pirildar S et al; Psychopharmacol Bull 37 (3): 127-34 (2003)
EXPTL Therapy: This randomized, prospective, double-blind study evaluated the efficacy and tolerability of moclobemide, a reversible, selective inhibitor of monoamine oxidase-A, in reducing the frequency and severity of hot flashes. Thirty postmenopausal women were enrolled, and 28 were allocated to 5 weeks of treatment with moclobemide 150 mg (group 1, n = 10), moclobemide 300 mg (group 2, n = 11), or placebo (group 3, n = 9). Data on hot flashes were recorded in a daily diary. Mean reductions in the hot flash severity score were 24.4% in the placebo group, 69.8% in group 1, and 35.0% in group 2.
PMID:12665046 Tarim E et al; Adv Ther 19 (6): 258-65 (2002)
EXPTL Therapy: In a double-blind placebo controlled study, 12 male outpatients suffering from psychogenic erectile dysfunction without any other psychiatric disorder were investigated. Based on comprehensive diagnosis before the beginning of the study, organic factors relevant for sexual function were excluded. The treatment period was 8 weeks. Half the patients received 450 mg moclobemide during the first week, and 600 mg afterwards; the others received placebo. Apart from assessment of erectile function by means of the Clinical Global Impression (CGI) scale, nocturnal erections were measured under polysomnographic control at baseline and at the end of the treatment period. The evaluation of the CGI scale revealed a clearly stronger improvement under moclobemide compared to placebo during the study period. The therapeutic efficacy found on the subjective level had no clear correlate on the neurophysiological level. No alterations of nocturnal erectile parameters were obvious under treatment; neither were clinically relevant alterations found regarding sleep EEG parameters. The medication was well tolerated without serious adverse events. The findings support the hypothesis that moclobemide has a specific effect on /psychogenic/ erectile dysfunction.
PMID:11465638 Mann K et al; Psychopharmacology (Berl) 156 (1): 86-91 (2001)
Moclobemide represents a new class of drug, the so-called RIMA compounds--reversible inhibitors of MAO-A. Unlike classical monoamine oxidase (MAO) inhibitors, moclobemide is devoid of hepatotoxicity and has only a slight potentiating effect on the hypertensive action of tyramine; treatment does not require a tyramine-restricted diet. Studies comparing moclobemide with tricyclic antidepressants (TCAs) indicate that moclobemide is significantly better tolerated than TCAs and slightly less well tolerated than placebo.
Amrein R et al; Can J Psychiatry 37 (Suppl 1): 7-11 (1992)
Safety aspects were compared in 2203 patients given moclobemide and 1214 who received other antidepressants or placebo. A total of 2294 adverse events were reported by patients on moclobemide, mainly subjective symptoms (28.6%). Adverse events such as dry mouth, tremor, sweating, dizziness and constipation occurred much more frequently among 681 patients treated with various tricyclic antidepressants than in the 694 moclobemide patients with whom they were compared.
PMID:2248078 Moll E, Hetzel W; Acta Psychiatr Scand Suppl 360: 69-70 (1990)
Because moclobemide is partially metabolized by the polymorphic isozymes CYP2C19 and CYP2D6, plasma concentrations of this medication may be affected in patients with genetically or drug-induced poor metabolism. Approximately 2% of whites and 15% of Asians can be genetically phenotyped as slow metabolizers with respect to oxidative hepatic metabolism. In slow metabolizer subjects, the area under the concentration-time curve (AUC) was found to be 1.5 times greater than in extensive metabolizer subjects for the same dose of moclobemide. This increase is within the normal range of variation (up to two-fold) typically seen in patients.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1932
Concurrent consumption of tyramine-rich food with irreversible monoamine inhibitors may cause sudden and severe hypertensive reactions; since moclobemide is a reversible inhibitor of MAO-A (RIMA), dietary restriction may not be necessary; consumption of up to 100 mg of tyramine with 600 mg of moclobemide per day is not expected to cause problems; potential hypertensive reactions may be minimized if moclobemide is taken after meals.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1933
Moclobemide had inconsistent effects on the blood pressure of hypertensive patients during clinical trials; careful monitoring is important, especially during initial titration.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1933
For more Drug Warnings (Complete) data for MOCLOBEMIDE (12 total), please visit the HSDB record page.
For the treatment of major depressive disorder and bipolar disorder.
A selective, reversible inhibitor of monoamine oxidase (MAO) which increases the. Besides its presence in sympathetic nerves, there is an abundant evidence that MAO-A is localized in noradrenergic neurons in the locus coeruleus and MAO-B is closely associated with serotonergic neurons of the raphe nucleus.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Monoamine Oxidase Inhibitors
A chemically heterogeneous group of drugs that have in common the ability to block oxidative deamination of naturally occurring monoamines. (From Gilman, et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed, p414) (See all compounds classified as Monoamine Oxidase Inhibitors.)
N06AG02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AG - Monoamine oxidase a inhibitors
N06AG02 - Moclobemide
Absorption
Well absorbed from the gastrointestinal tract (> 95%). The presence of food reduces the rate but not the extent of absorption. Hepatic first-pass metabolism reduces bioavailability to about 56% following administration of one dose, but increases to 90% with steady-state dosing as a result of saturation of the first pass effect. Peak plasma concentrations are reached within 0.3 - 1 hours following oral administration with a terminal half-life of 1.6h.
Route of Elimination
Moclobemide is almost completely renally excreted.
Volume of Distribution
1-1.5 L/Kg
Clearance
Clearance of 30-78 L/h, mainly excreted in urine.
Moclobemide is readily absorbed (>95%) through the gastrointestinal tract. Within 1 to 2 hours of oral use, a peak plasma level of about 1 ug/mL is reached. It is protein bound to the extent of 50%.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 656
Small quantities of moclobemide are distributed into human breast milk.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1932
Well absorbed from the gastrointestinal tract. The presence of food reduces the rate but not the extent of absorption. Absolute bioavailability ranges from approximately 55% following administration of single doses of moclobemide to 90% following multiple dosing, due to the hepatic first pass effect.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1932
Elimination: Renal, as metabolites. Less than 1% of an administered dose of moclobemide is eliminated unchanged.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1932
The excretion of moclobemide and its major metabolites in human breast milk was studied in 6 lactating women (aged 24-36 yr) who received a single dose of 300 mg moclobemide in oral tablet form. Moclobemide and /3-keto-meclobemide/ (Ro-12-8095) were measured in milk and plasma samples. /Moclobemide-N'-oxide/ (Ro-12-5637) was only detected in plasma. The concentrations of moclobemide and Ro-12-8095 in milk were highest at 3 hr after moclobemide administration and the drug and metabolite were not detectable after 12 hr. The percentages of the dose excreted as moclobemide and Ro-12-8095 were 0.057+/-0.02% and 0.031+/-0.011%, respectively.
PMID:2297459 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1380057 Pons G et al; Br J Clin Pharmacol 29 (Jan): 27-31(1990)
Moclobemide is almost completely metabolized in the liver by Cytochrome P450 2C19 and 2D6. Moclobemide is a substrate of CYP2C19. Although it acts as an inhibitor of CYP1A2, CYP2C19, and CYP2D6.
The role of mephenytoin oxidation polymorphism in the metabolism of moclobemide was studied in 15 healthy subjects (ages 23-27 yr), including 7 poor metabolizers and 8 extensive metabolizers of S-mephenytoin, who received a single dose of 300 mg moclobemide and multiple doses of 600 mg/day moclobemide. Poor metabolizers of S-mephenytoin had lower moclobemide clearance (median single dose 16.1 vs 43.2 L/hr) and longer half-life (median single dose 4 vs 1.8 hr) compared with extensive metabolizers. Plasma levels of a metabolite formed by C-hydroxylation were lower in poor metabolizers. Moclobemide thus partially underwent oxidative metabolism via polymorphic CYP2C19. Changes in metabolic indexes were compatible with reversible inhibition of oxidation by way of CYP2C19, CYP2D6, and CYP1A2. It was concluded that there is a cosegregation between moclobemide clearance and mephenytoin oxidation polymorphism.
PMID:7781267 Gram LF et al; Clin Pharmacol Ther 57 (Jun): 670-7 (1995)
Moclobemide appears to be eliminated (after first-pass hepatic metabolism) by first-order kinetics, resulting in urinary excretion of the monoamine metabolites homovanillic acid (HAV), dihydroxyphenylacetic acid (DOPAC), 3-methoxy-4-hydroxy-phenyl glycol (DOPEG), and 5-hydroxyindoleacetic acid (5-HIAA).
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 656
1-2 hours (4 hours in cirrhotic patients); metabolites are renally excreted
Elimination: 1.5 hours (4 hours in cirrhotic patients).
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1932
The mechanism of action of moclobemide involves the selective, reversible inhibition of MAO-A. This inhibition leads to a decrease in the metabolism and destruction of monoamines in the neurotransmitters. This results in an increase in the monoamines, relieving depressive symptoms.
The exact mechanism of antidepressant effect is unknown; however, it is established that the activity of the enzyme monoamine oxidase (MAO) is inhibited. MAO subtypes A and B are involved in the metabolism of serotonin and catecholamine neurotransmitters, such as norepinephrine and dopamine. Moclobemide, as a selective MAO inhibitor, preferentially inhibits monoamine oxidase-A (MAO-A) and, to a lesser extent, monoamine oxidase-B (MAO-B) (approximately 80% inhibition of MAO-A and 20% to 30% inhibition of MAO-B, 300 mg). Reduced MAO activity results in an increased concentration of serotonin and catecholamine neurotransmitters in storage sites throughout the central nervous system (CNS) and sympathetic nervous system. This increased availability of one or more monoamines has been thought to be the basis for the antidepressant activity of MAO inhibitors. MAO-A inhibition by moclobemide is short-acting (maximum 24 hours) and reversible (transient binding to MAO-A). In contrast, some other MAO inhibitors (phenelzine, tranylcypromine) are non-selective, long-acting, and irreversible in their binding to MAO-A and MAO-B.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1931

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

ANALYTICAL

ABOUT THIS PAGE
48
PharmaCompass offers a list of Moclobemide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Moclobemide manufacturer or Moclobemide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Moclobemide manufacturer or Moclobemide supplier.
PharmaCompass also assists you with knowing the Moclobemide API Price utilized in the formulation of products. Moclobemide API Price is not always fixed or binding as the Moclobemide Price is obtained through a variety of data sources. The Moclobemide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Moclobemide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Moclobemide, including repackagers and relabelers. The FDA regulates Moclobemide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Moclobemide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Moclobemide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Moclobemide supplier is an individual or a company that provides Moclobemide active pharmaceutical ingredient (API) or Moclobemide finished formulations upon request. The Moclobemide suppliers may include Moclobemide API manufacturers, exporters, distributors and traders.
click here to find a list of Moclobemide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Moclobemide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Moclobemide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Moclobemide GMP manufacturer or Moclobemide GMP API supplier for your needs.
A Moclobemide CoA (Certificate of Analysis) is a formal document that attests to Moclobemide's compliance with Moclobemide specifications and serves as a tool for batch-level quality control.
Moclobemide CoA mostly includes findings from lab analyses of a specific batch. For each Moclobemide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Moclobemide may be tested according to a variety of international standards, such as European Pharmacopoeia (Moclobemide EP), Moclobemide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Moclobemide USP).
