
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Weekly News Recap #Phispers
US Medicaid
NA
Finished Drug Prices
NA
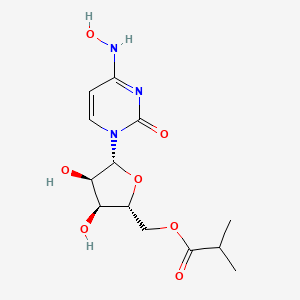

1. ((2r,3s,4r,5r)-3,4-dihydroxy-5-((4z)-4-(hydroxyimino)-2-oxo-3,4- Dihydropyrimidin-1(2h)-yl)oxolan-2-yl)methyl 2-methylpropanoate
2. Lagevrio
3. Mk-4482
4. Molnupiravir
1. Eidd 2801
2. Eidd2801
3. Molnupiravir
4. 2349386-89-4
5. Mk-4482
6. Molnupiravir [inn]
7. 2492423-29-5
8. Molnupiravir [usan]
9. Molnupiravir [who-dd]
10. Ya84ki1vew
11. Eidd 1931-isopropyl Ester
12. Uridine, 4-oxime, 5'-(2-methylpropanoate), (4z)-
13. N4-hydroxycytidine, 5'-isopropyl Ester
14. ((2r,3s,4r,5r)-3,4-dihydroxy-5-((4z)-4-(hydroxyimino)-2-oxo-3,4- Dihydropyrimidin-1(2h)-yl)oxolan-2-yl)methyl 2-methylpropanoate
15. ((2r,3s,4r,5r)-3,4-dihydroxy-5-(4-(hydroxyamino)-2-oxopyrimidin-1(2h)-yl)tetrahydrofuran-2-yl)methyl Isobutyrate
16. [(2r,3s,4r,5r)-3,4-dihydroxy-5-[4-(hydroxyamino)-2-oxopyrimidin-1-yl]oxolan-2-yl]methyl 2-methylpropanoate
17. Lagevrio
18. Who 11853
19. ((2r,3s,4r,5r)-3,4-dihydroxy-5-(4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2h)-yl)tetrahydrofuran-2-yl)methyl Isobutyrate
20. Molnupiravirum
21. Unii-ya84ki1vew
22. Pro-eidd-1931
23. Molnupiravir [jan]
24. Eidd-2801(molnupiravir)
25. Chembl4650320
26. Eidd 1931 5'-isopropylester
27. Gtpl10737
28. Med.21724, Compound 182
29. Chebi:180653
30. Bdbm429508
31. Dtxsid501028058
32. Bcp32744
33. Eidd-1931 Isopropyl Ester
34. Ex-a3432
35. Mfcd32663515
36. Mk4482
37. S8969
38. At13078
39. N(4)-hydroxycytidine 5'-isopropylester
40. Ac-35171
41. As-84465
42. Beta-d-n4 Hydroxycytidine-5'-isopropyl Ester
43. Beta-d-n(4)-hydroxycytidine-5'-isopropyl Ester
44. A936190
45. .beta.-d-n4 Hydroxycytidine-5'-isopropyl Ester
46. N-hydroxy-5'-o-(2-methylpropanoyl)-3,4-dihydrocytidine
47. Eidd 2801; Eidd2801; Uridine, 4-oxime, 5'-(2-methylpropanoate
48. ((2r,3s,4r,5r)-3,4-dihydroxy-5-((e)-4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2h)-yl)tetrahydrofuran-2-yl)methyl Isobutyrate
49. ((2r,3s,4r,5r)-3,4-dihydroxy-5-((z)-4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2h)-yl)tetrahydrofuran-2-yl)methyl Isobutyrate
50. [(2r,3s,4r,5r)-3,4-dihydroxy-5-[(4e)-4-(hydroxyimino)-2-oxo-1,2,3,4-tetrahydropyrimidin-1-yl]oxolan-2-yl]methyl 2-methylpropanoate
51. {(2r,3s,4r,5r)-3,4-dihydroxy-5-[4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2h)-yl]tetrahydrofuran-2-yl}methyl 2-methylpropanoate
| Molecular Weight | 329.31 g/mol |
|---|---|
| Molecular Formula | C13H19N3O7 |
| XLogP3 | -0.8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 329.12229995 g/mol |
| Monoisotopic Mass | 329.12229995 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 534 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
[N4-hydroxycytidine] and its prodrug molnupiravir are being studied for its activity against a number of viral infections including influenza, MERS-CoV, and SARS-CoV-2. Molnupiravir is approved in the UK for reducing the risk of hospitalization and death in mild to moderate COVID-19 cases for patients at increased risk of severe disease (eg. with obesity, diabetes mellitus, heart disease, or are over 60 years old).
Treatment of Coronavirus disease 2019 (COVID-19)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Absorption
After an 800 mg oral dose of molnupiravir every 12 hours, the active compound (N4-hydroxycytidine) reaches a Cmax of 2970 ng/mL, with a Tmax of 1.5 hours, and an AUC0-12h of 8360 h\*ng/mL.
Route of Elimination
3% of an oral molnupiravir dose is eliminated in the urine as the active metabolite N4-hydroxycytidine.
Molnupiravir is hydrolyzed to [N4-hydroxycytidine], which distributes into tissues. Once inside cells, N4-hydroxycytidine is phosphorylated to the 5'-triphosphate form.
The half life of the active metabolite, N4-hydroxycytidine, is 3.3 hours.
Molnupiravir is hydrolyzed _in vivo_ to N4-hydroxycytidine, which is phosphorylated in tissue to the active 5-triphosphate form, and incorporated into the genome of new virions, resulting in the accumulation of inactivating mutations, known as viral error catastrophe. A [remdesivir] resistant mutant mouse hepatitis virus has also been shown to have increased sensitivity to N4-hydroxycytidine.

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?