
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
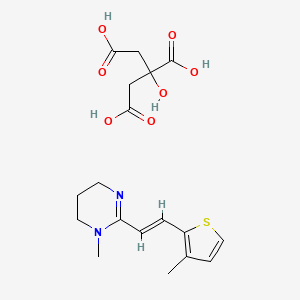

1. 69525-81-1
2. Morantel Citrate Salt
3. Morantel (citrate)
4. Vc8z4ss5qx
5. 2-hydroxypropane-1,2,3-tricarboxylic Acid;1-methyl-2-[(e)-2-(3-methylthiophen-2-yl)ethenyl]-5,6-dihydro-4h-pyrimidine
6. (e)-1-methyl-2-(2-(3-methylthiophen-2-yl)vinyl)-1,4,5,6-tetrahydropyrimidine 2-hydroxypropane-1,2,3-tricarboxylate
7. Unii-vc8z4ss5qx
8. Exhelm
9. Einecs 274-028-9
10. Exhelm (tn)
11. Mls004712037
12. Spectrum1503931
13. Schembl2777336
14. Chembl1330312
15. Schembl22320735
16. Hms502l08
17. Dtxsid90219780
18. Morantel Citrate [mart.]
19. Hms1922m08
20. Hms2093m11
21. Pharmakon1600-01503931
22. Ex-a1463
23. Ccg-39377
24. Nsc758647
25. Akos024255719
26. Hs-0101
27. Ncgc00095083-01
28. Ncgc00095083-02
29. (e)-1,4,5,6-tetrahydro-1-methyl-2-(2-(3-methyl-2-thienyl)vinyl)pyrimidinediylium Hydrogen Citrate
30. Ac-32489
31. Smr003475008
32. Hy-108387
33. Cs-0028552
34. D08231
35. Sr-01000721901-2
36. Q27291761
| Molecular Weight | 412.5 g/mol |
|---|---|
| Molecular Formula | C18H24N2O7S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 176 |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 500 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
ABOUT THIS PAGE
