
Synopsis
Synopsis
0
VMF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
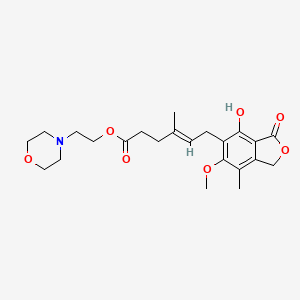

1. Cellcept
2. Mofetil Hydrochloride, Mycophenolate
3. Mofetil, Mycophenolate
4. Mycophenolate Mofetil Hydrochloride
5. Mycophenolate Sodium
6. Mycophenolate, Sodium
7. Mycophenolic Acid
8. Mycophenolic Acid Morpholinoethyl Ester
9. Myfortic
10. Rs 61443
11. Rs-61443
12. Rs61443
13. Sodium Mycophenolate
1. 128794-94-5
2. Cellcept
3. 115007-34-6
4. Rs 61443
5. Myfenax
6. Rs-61443
7. Tm-mmf
8. Myclausen
9. Mycophenylate Mofetil
10. Mycophenolic Acid Morpholinoethyl Ester
11. Mycophenolatemofetil
12. Mycophenolate Mofetil Teva
13. Chebi:8764
14. 2-morpholin-4-ylethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-2-benzofuran-5-yl)-4-methylhex-4-enoate
15. Cellcept (tn)
16. (e)-2-morpholinoethyl 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate
17. Nsc-724229
18. Nsc-758905
19. 2-morpholinoethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoate
20. Mycophenolate Mofetil (cellcept)
21. 9242ecw6r0
22. 2-(morpholin-4-yl)ethyl (4e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoate
23. 2-morpholinoethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate
24. 4-hexenoic Acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, 2-(4-morpholinyl)ethyl Ester, (e)-
25. Munoloc
26. Dsstox_cid_3340
27. Dsstox_rid_76982
28. Dsstox_gsid_23340
29. Mycophenolate Mofetil 100 Microg/ml In Acetonitrile
30. Mycophenolic Acid 2-(4-morpholinyl)ethyl Ester
31. Nsc 758905
32. 2-morpholin-4-ylethyl (4e)-6-[4-hydroxy-7-methyl-6-(methyloxy)-3-oxo-1,3-dihydro-2-benzofuran-5-yl]-4-methylhex-4-enoate
33. 2-morpholinoethyl 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate
34. 4-hexenoic Acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, 2-(4-morpholinyl)ethyl Ester, (4e)-
35. Smr002544686
36. Mmf Cellcept(tm)
37. Cas-128794-94-5
38. Hsdb 7436
39. Me-mpa
40. Sr-05000001485
41. Mycophenolate Mofetil [usan]
42. Arzip
43. Unii-9242ecw6r0
44. Mycophenolatmofetil
45. Mycophenolate Mofetil [usan:usp]
46. Ncgc00159459-02
47. (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-isobenzofuran-5-yl)-4-methyl-4-hexenoic Acid 2-(4-morpholinyl)ethyl Ester
48. Mycophenolate-mofetil
49. Mofetil Mycophenolate
50. Mfcd00867568
51. R-99
52. Schembl4195
53. Chembl1456
54. Mls003915627
55. Mls004774133
56. Mls006011929
57. Schembl218782
58. Gtpl6831
59. Dtxsid3023340
60. Chebi:93612
61. Mycophenolate Mofetil (jan/usp)
62. Hms2090a03
63. Pharmakon1600-01504567
64. Mycophenolate Mofetil [jan]
65. 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-isobenzofuran-5-yl)-4-methyl-4-hexenoic Acid 2-(4-morpholinyl)ethyl Ester
66. Act01993
67. Albb-027273
68. Hy-b0199
69. Mycophenolate Mofetil [hsdb]
70. Tox21_111686
71. Bbl029073
72. Bdbm50248299
73. Mycophenolate Mofetil [vandf]
74. Nsc724229
75. Nsc758905
76. S1501
77. Stl146382
78. Zinc21297660
79. Mycophenolate Mofetil [mart.]
80. Akos005720900
81. Mycophenolate Mofetil [usp-rs]
82. Mycophenolate Mofetil [who-dd]
83. Tox21_111686_1
84. Ac-1562
85. Bcp9000969
86. Ccg-213315
87. Db00688
88. Ks-1209
89. Nsc 724229
90. Rs61443
91. Mycophenolate Mofetil [ema Epar]
92. Mycophenolate Mofetil, >=98% (hplc)
93. Ncgc00159459-03
94. Ncgc00159459-04
95. 2-morpholinoethyl (e)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1h-isobenzofuran-5-yl)-4-methyl-hex-4-enoate
96. Bm164622
97. Ls-15013
98. Smr004703518
99. Mycophenolate Mofetil [orange Book]
100. Mycophenolate Mofetil [ep Monograph]
101. M2387
102. Mycophenolate Mofetil [usp Monograph]
103. Mycophenolate Mofetil For Peak Identification
104. Sw219893-1
105. C07908
106. D00752
107. W18801
108. Ab01274794-01
109. Ab01274794-02
110. Ab01274794_03
111. Ab01274794_04
112. 007m346
113. A803280
114. A805863
115. A888971
116. J-005626
117. Q-101316
118. Q4567614
119. Sr-05000001485-1
120. Sr-05000001485-2
121. Brd-k92428153-001-01-0
122. Mycophenolic Acid 2-(4-morpholinyl)ethyl Ester [mi]
123. Mycophenolate Mofetil, European Pharmacopoeia (ep) Reference Standard
124. Mycophenolate Mofetil, United States Pharmacopeia (usp) Reference Standard
125. Mycophenolate Mofetil For Peak Identification, European Pharmacopoeia (ep) Reference Standard
126. Mycophenolate Mofetil, Pharmaceutical Secondary Standard; Certified Reference Material
127. (1s,3r,4s)-2-amino-9-[4-(benzyloxy)-3-(benzyloxymethyl)-2-methylidene-cyclopentyl]-3h-purin-6-one
128. (e)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic Acid 2-(4-morpholinyl)ethyl Ester
129. 140401-05-4
130. 2-(4-morpholino)ethyl (e)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl)-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate
131. 2-(4-morpholinyl)ethyl (e)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate
132. 2-morpholin-4-ylethyl (e)-6-(6-methoxy-7-methyl-4-oxidanyl-3-oxidanylidene-1h-2-benzofuran-5-yl)-4-methyl-hex-4-enoate
133. 4-hexenoic Acid,3-dihydro-4-hydroxy-6-methoxy-7-methyl -3-oxo-5-isobenzofuranyl)-4-methyl-, 2-(4-morpholinyl)ethyl Ester, (4e)
134. 6-((7-hydroxy-5-methoxy-4-methyl-1-oxo-3h-isobenzofuran-6-yl))-4-methyl-hex-4-enoic Acid 2-morpholinoethyl Ester
135. 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic Acid 2-(4-morpholinyl)ethyl Ester
136. Morpholinoethyl (e)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate
137. Morpholinoethyl 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)- 4-methylhex-4-enoate
138. Morpholinoethyl E-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate
139. Morpholinoethyl E-6-(1,3-dihydro-4-hydroxy-6-methoxy-7methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate
| Molecular Weight | 433.5 g/mol |
|---|---|
| Molecular Formula | C23H31NO7 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Exact Mass | 433.21005233 g/mol |
| Monoisotopic Mass | 433.21005233 g/mol |
| Topological Polar Surface Area | 94.5 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 646 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Cellcept |
| PubMed Health | Mycophenolate |
| Drug Classes | Immune Suppressant |
| Drug Label | CellCept (mycophenolate mofetil) is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6... |
| Active Ingredient | Mycophenolate mofetil; Mycophenolate mofetil hydrochloride |
| Dosage Form | Injectable; Tablet; Capsule; Suspension |
| Route | Injection; Oral |
| Strength | 500mg/vial; 250mg; 200mg/ml; 500mg |
| Market Status | Prescription |
| Company | Roche Palo |
| 2 of 4 | |
|---|---|
| Drug Name | Mycophenolate mofetil |
| PubMed Health | Mycophenolate Mofetil (Oral route) |
| Drug Classes | Immune Suppressant |
| Drug Label | Mycophenolate mofetil is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor. The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1, 3-dih... |
| Active Ingredient | Mycophenolate mofetil |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Apotex; Accord Hlthcare; Alkem Labs; Sandoz; Roxane; Strides Pharma; Teva Pharms; Dr Reddys Labs; Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Cellcept |
| PubMed Health | Mycophenolate |
| Drug Classes | Immune Suppressant |
| Drug Label | CellCept (mycophenolate mofetil) is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6... |
| Active Ingredient | Mycophenolate mofetil; Mycophenolate mofetil hydrochloride |
| Dosage Form | Injectable; Tablet; Capsule; Suspension |
| Route | Injection; Oral |
| Strength | 500mg/vial; 250mg; 200mg/ml; 500mg |
| Market Status | Prescription |
| Company | Roche Palo |
| 4 of 4 | |
|---|---|
| Drug Name | Mycophenolate mofetil |
| PubMed Health | Mycophenolate Mofetil (Oral route) |
| Drug Classes | Immune Suppressant |
| Drug Label | Mycophenolate mofetil is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor. The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1, 3-dih... |
| Active Ingredient | Mycophenolate mofetil |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 250mg; 500mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Apotex; Accord Hlthcare; Alkem Labs; Sandoz; Roxane; Strides Pharma; Teva Pharms; Dr Reddys Labs; Mylan |
Anti-Inflammatory Agents, Non-Steroidal, Antineoplastic Agents, Dermatologic Agents, Enzyme Inhibitors, Immunosuppressive Agents
National Library of Medicine, SIS; ChemIDplus Record for Mycophenolate Mofetil (128794-94-5), MESH Heading. Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION: Immunosuppressant
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1132
Mycophenolate is indicated, in combination with cyclosporine and corticosteroids, for prevention of rejection of allogeneic cardiac, hepatic and renal transplants. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2141
Mycophenolate is indicated for the treatment of lupus nephritis. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2141
Severe GI bleeding (requiring hospitalization) has occurred in 3, 1.7, or 5.4% of renal, cardiac, or hepatic transplant recipients, respectively, receiving 3-g daily dosages of mycophenolate mofetil in clinical studies. Because mycophenolate mofetil has been associated rarely with an increased incidence of adverse GI effects (e.g., ulceration, hemorrhage, perforation), the drug should be administered with caution in patients with active serious GI disease.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3676
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2142
Severe neutropenia (i.e., absolute neutrophil counts (ANC) of less than 500/cu mm) has been reported in up to 2, 2.8, or 3.6% of renal, cardiac, or hepatic allograft recipients, respectively, receiving 3-g daily dosages of mycophenolate mofetil. Neutropenia has been observed most frequently between 31-180 days post-transplant in patients receiving immunosuppressive therapy for the prevention of rejection of kidney, heart, or liver allograft. Neutropenia may be related to mycophenolate mofetil, concomitant therapies, viral infection, or a combination of these causes.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3676
Potential for the development of lymphoma and other malignancies, particularly of the skin, which may result from immunosuppression. Because of the increased risk for skin cancer, patients should be advised to limit their exposure to sunlight or other UV light by wearing protective clothing and using sunscreen with a high protection factor. Lymphoproliferative disease or lymphoma occurred in 0.4-1% of allograft recipients receiving mycophenolate mofetil in conjunction with other immunosuppressive agents in clinical studies. Non-melanoma skin carcinoma was reported in 1.6-4.2% of patients while other types of malignancy were reported in 0.7-2.1% of patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3675
For more Drug Warnings (Complete) data for MYCOPHENOLATE MOFETIL (15 total), please visit the HSDB record page.
Mycophenolate mofetil is indicated for the prophylaxis of organ rejection in patients undergoing allogeneic renal, hepatic, or cardiac transplants. It should be used with cyclosporine and corticosteroids. Mycophenolate mofetil may also be used off-label as a second-line treatment for autoimmune hepatitis that has not responded adequately to first-line therapy. Other off-label uses of this drug include lupus-associated nephritis and dermatitis in children.
FDA Label
Myfenax is indicated in combination with ciclosporin and corticosteroids for the prophylaxis of acute transplant rejection in patients receiving allogeneic renal, cardiac or hepatic transplants.
Mycophenolate mofetil Teva is indicated in combination with ciclosporin and corticosteroids for the prophylaxis of acute transplant rejection in patients receiving allogeneic renal, cardiac or hepatic transplants.
CellCept is indicated in combination with ciclosporin and corticosteroids for the prophylaxis of acute transplant rejection in patients receiving allogeneic renal, cardiac or hepatic transplants.
Myclausen is indicated in combination with ciclosporin and corticosteroids for the prophylaxis of acute transplant rejection in patients receiving allogeneic renal, cardiac or hepatic transplants.
Mycophenolate mofetil is a prodrug of mycophenolic acid (MPA). The active form of mycophenolate, MPA, prevents the proliferation of immune cells and the formation of antibodies that cause transplant rejection. The above effects lead to higher rates of successful transplantation, avoiding the devastating effects of graft rejection.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Antibiotics, Antitubercular
Substances obtained from various species of microorganisms that are, alone or in combination with other agents, of use in treating various forms of tuberculosis; most of these agents are merely bacteriostatic, induce resistance in the organisms, and may be toxic. (See all compounds classified as Antibiotics, Antitubercular.)
L04AA06
L04AA06
L04AA06
L04AA06
Absorption
Mycophenolate mofetil is rapidly absorbed in the small intestine. The maximum concentration of its active metabolite, MPA, is attained 60 to 90 minutes following an oral dose. The average bioavailability of orally administered mycophenolate mofetil in a pharmacokinetic study of 12 healthy patients was 94%. In healthy volunteers, the Cmax of mycophenolate mofetil was 24.5 (9.5)g/mL. In renal transplant patients 5 days post-transplant, Cmax was 12.0 (3.82) g/mL, increasing to 24.1 (12.1)g/mL 3 months after transplantation. AUC values were 63.9 (16.2) gh/mL in healthy volunteers after one dose, and 40.8 (11.4) gh/mL, and 65.3 (35.4)gh/mL 5 days and 3 months after a renal transplant, respectively. The absorption of mycophenolate mofetil is not affected by food.
Route of Elimination
A small amount of drug is excreted as MPA in the urine (less than 1%). When mycophenolate mofetil was given orally in a pharmacokinetic study, it was found to be 93% excreted in urine and 6% excreted in feces. Approximately 87% of the entire administered dose is found to be excreted in the urine as MPAG, an inactive metabolite.
Volume of Distribution
The volume of distribution of mycophenolate mofetil is 3.6 (1.5) to 4.0 (1.2) L/kg.
Clearance
Plasma clearance of mycophenolate mofetil is 193 mL/min after an oral dose and 177 (31) mL/min after an intravenous dose.
/Absorption/ is rapid and extensive after oral administration.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2142
In 12 healthy volunteers, the mean absolute bioavailability of oral mycophenolate mofetil relative to intravenous mycophenolate mofetil (based on MPA AUC) was 94%. The area under the plasma-concentration time curve (AUC) for MPA appears to increase in a dose-proportional fashion in renal transplant patients receiving multiple doses of mycophenolate mofetil up to a daily dose of 3 g.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 2752
Protein binding: To plasma albumin: High (97% for mycophenolic acid (MPA) at concentration ranges normally seen in stable renal transplant patients). At higher mycophenolic acid glucuronide (MPAG) concentrations (e.g., in patients with renal impairment or delayed graft function), binding of MPA may be decreased as a result of competition between MPA and MPAG for binding sites.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2142
The mean (+/-SD) apparent volume of distribution of MPA in 12 healthy volunteers is approximately 3.6 (+/-1.5) and 4.0 (+/-1.2) L/kg following intravenous and oral administration, respectively. MPA, at clinically relevant concentrations, is 97% bound to plasma albumin. MPAG is 82% bound to plasma albumin at MPAG concentration ranges that are normally seen in stable renal transplant patients; however, at higher MPAG concentrations (observed in patients with renal impairment or delayed renal graft function), the binding of MPA may be reduced as a result of competition between MPAG and MPA for protein binding. Mean blood to plasma ratio of radioactivity concentrations was approximately 0.6 indicating that MPA and MPAG do not extensively distribute into the cellular fractions of blood.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 2752
For more Absorption, Distribution and Excretion (Complete) data for MYCOPHENOLATE MOFETIL (9 total), please visit the HSDB record page.
After both oral and intravenous administration mycophenolate mofetil is entirely metabolized by liver carboxylesterases 1 and 2 to mycophenolic acid (MPA), the active parent drug. It is then metabolized by the enzyme glucuronyl transferase, producing the inactive phenolic glucuronide of MPA (MPAG). The glucuronide metabolite is important, as it is then converted to MPA through enterohepatic recirculation. Mycophenolate mofetil that escapes metabolism in the intestine enters the liver via the portal vein and is transformed to pharmacologically active MPA in the liver cells.N-(2-carboxymethyl)-morpholine, N-(2-hydroxyethyl)-morpholine, and the N-oxide portion of N-(2-hydroxyethyl)-morpholine are additional metabolites of MMF occurring in the intestine as a result of liver carboxylesterase 2 activity. UGT1A9 and UGT2B7 in the liver are the major enzymes contributing to the metabolism of MPA in addition to other UGT enzymes, which also play a role in MPA metabolism. The four major metabolites of MPA are 7-O-MPA--glucuronide (MPAG, inactive), MPA acyl-glucuronide (AcMPAG), produced by uridine 5-diphosphate glucuronosyltransferases (UGT) activities, 7-O-MPA glucoside produced via UGT, and small amounts 6-O-des-methyl-MPA (DM-MPA) via CYP3A4/5 and CYP2C8 enzymes.
Following oral and intravenous dosing, mycophenolate mofetil undergoes complete metabolism to MPA /mycophenolic acid/, the active metabolite. Metabolism to MPA occurs presystemically after oral dosing. MPA is metabolized principally by glucuronyl transferase to form the phenolic glucuronide of MPA (MPAG) which is not pharmacologically active. In vivo, MPAG is converted to MPA via enterohepatic recirculation. The following metabolites of the 2- hydroxyethyl-morpholino moiety are also recovered in the urine following oral administration of mycophenolate mofetil to healthy subjects: N-(2-carboxymethyl)-morpholine, N-(2- hydroxyethyl)-morpholine, and the N-oxide of N-(2-hydroxyethyl)-morpholine.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 2752
The average apparent half-life of mycophenolate mofetil is 17.9 (6.5) hours after oral administration and 16.6 (5.8) hours after intravenous administration.
For mycophenolic acid (MPA):Mean apparent: Approximately 17.9 hours after oral administration and 16.6 hours after intravenous administration.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2142
Mean (+/-SD) apparent half-life and plasma clearance of MPA are 17.9 (+/-6.5) hours and 193 (+/-48) mL/min following oral administration and 16.6 (+/-5.8) hours and 177 (+/-31) mL/min following intravenous administration, respectively.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 2752
The active metabolite of mycophenolate, mycophenolic acid, prevents T-cell and B-cell proliferation and the production of cytotoxic T-cells and antibodies. Lymphocyte and monocyte adhesion to endothelial cells of blood vessels that normally part of inflammation is prevented via the glycosylation of cell adhesion molecules by MPA. MPA inhibits de novo purine biosynthesis (that promotes immune cell proliferation) by inhibiting inosine 5-monophosphate dehydrogenase enzyme (IMPDH), with a preferential inhibition of IMPDH II. IMPDH normally transforms inosine monophosphate (IMP) to xanthine monophosphate (XMP), a metabolite contributing to the production of guanosine triphosphate (GTP). GTP is an important molecule for the synthesis of ribonucleic acid (RNA), deoxyribonucleic acid (DNA), and protein. As a result of the above cascade of effects, mycophenolate mofetil reduces de-novo production of guanosine nucleotides, interfering with the synthesis of DNA, RNA, and protein required for immune cell production. Further contributing to the above anti-inflammatory effects, MMF depletes tetrahydrobiopterin, causing the decreased function of inducible nitric oxide synthase enzyme, in turn decreasing the production of peroxynitrite, a molecule that promotes inflammation.
As a potent, selective, noncompetitive, and reversible, inhibitor of inosine monophosphate dehydrogenase (IMPDH), mycophenolic acid (MPA), the active metabolite /of mycophenolate mofetil/, inhibits the de novo synthesis pathway of guanosine nucleotides without being incorporated into DNA. Because T and B lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, while other cell types can utilize salvage pathways, MPA has potent cytostatic effects on lymphocytes. MPA inhibit proliferative responses of T and B lymphocytes to both mitogenic and allospecific stimulation. The addition of guanosine or deoxyguanosine reverses the cytostatic effects of MPA on lymphocytes. MPA also suppresses antibody formation by B lymphocytes. MPA prevents the glycosylation of lymphocytes and monocyte glycoproteins that are involved in intercellular adhesion of these cells to endothelial cells, and may inhibit recruitment of leukocytes into sites of inflammation and graft rejection. Mycophenolate mofetil dose not inhibit the early events in the activation of human peripheral blood mononuclear cells, such as the production of interleukin-1 and interleukin-2, but does block the coupling of these events to DNA synthesis and proliferation.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2141

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Regulatory Info :
Registration Country : India
Brand Name : Mycophenolate Mofetil
Dosage Form : DC Granules
Dosage Strength : 250MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Regulatory Info :
Registration Country : India
Brand Name : Mycophenolate Mofetil
Dosage Form : DC Granules
Dosage Strength : 500MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Regulatory Info : RX
Registration Country : USA
Brand Name : MYCOPHENOLATE MOFETIL
Dosage Form : CAPSULE;ORAL
Dosage Strength : 250MG
Packaging :
Approval Date : 2010-06-10
Application Number : 90055
Regulatory Info : RX
Registration Country : USA
Regulatory Info : DISCN
Registration Country : USA
Brand Name : MYCOPHENOLATE MOFETIL
Dosage Form : CAPSULE;ORAL
Dosage Strength : 250MG
Packaging :
Approval Date : 2009-12-22
Application Number : 90111
Regulatory Info : DISCN
Registration Country : USA
Regulatory Info : RX
Registration Country : USA
Brand Name : MYCOPHENOLATE MOFETIL
Dosage Form : TABLET;ORAL
Dosage Strength : 500MG
Packaging :
Approval Date : 2010-06-10
Application Number : 90456
Regulatory Info : RX
Registration Country : USA
Regulatory Info : RX
Registration Country : USA
Brand Name : MYCOPHENOLATE MOFETIL
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : 200MG/ML
Packaging :
Approval Date : 2023-08-29
Application Number : 212634
Regulatory Info : RX
Registration Country : USA
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Capsule
Dosage Strength : 250MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 500MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : MYCOPHENOLATE MOFETIL
Dosage Form : TABLET;ORAL
Dosage Strength : 500MG
Packaging :
Approval Date : 2010-07-16
Application Number : 90606
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : CELLCEPT
Dosage Form : TABLET
Dosage Strength : 500MG
Packaging : 50
Approval Date :
Application Number : 2237484
Regulatory Info : Prescription
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
35
PharmaCompass offers a list of Mycophenolate Mofetil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mycophenolate Mofetil manufacturer or Mycophenolate Mofetil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mycophenolate Mofetil manufacturer or Mycophenolate Mofetil supplier.
PharmaCompass also assists you with knowing the Mycophenolate Mofetil API Price utilized in the formulation of products. Mycophenolate Mofetil API Price is not always fixed or binding as the Mycophenolate Mofetil Price is obtained through a variety of data sources. The Mycophenolate Mofetil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Mycophenolate Mofetil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Mycophenolate Mofetil, including repackagers and relabelers. The FDA regulates Mycophenolate Mofetil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Mycophenolate Mofetil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Mycophenolate Mofetil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Mycophenolate Mofetil supplier is an individual or a company that provides Mycophenolate Mofetil active pharmaceutical ingredient (API) or Mycophenolate Mofetil finished formulations upon request. The Mycophenolate Mofetil suppliers may include Mycophenolate Mofetil API manufacturers, exporters, distributors and traders.
click here to find a list of Mycophenolate Mofetil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Mycophenolate Mofetil DMF (Drug Master File) is a document detailing the whole manufacturing process of Mycophenolate Mofetil active pharmaceutical ingredient (API) in detail. Different forms of Mycophenolate Mofetil DMFs exist exist since differing nations have different regulations, such as Mycophenolate Mofetil USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Mycophenolate Mofetil DMF submitted to regulatory agencies in the US is known as a USDMF. Mycophenolate Mofetil USDMF includes data on Mycophenolate Mofetil's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Mycophenolate Mofetil USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Mycophenolate Mofetil suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Mycophenolate Mofetil Drug Master File in Japan (Mycophenolate Mofetil JDMF) empowers Mycophenolate Mofetil API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Mycophenolate Mofetil JDMF during the approval evaluation for pharmaceutical products. At the time of Mycophenolate Mofetil JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Mycophenolate Mofetil suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Mycophenolate Mofetil Drug Master File in Korea (Mycophenolate Mofetil KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Mycophenolate Mofetil. The MFDS reviews the Mycophenolate Mofetil KDMF as part of the drug registration process and uses the information provided in the Mycophenolate Mofetil KDMF to evaluate the safety and efficacy of the drug.
After submitting a Mycophenolate Mofetil KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Mycophenolate Mofetil API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Mycophenolate Mofetil suppliers with KDMF on PharmaCompass.
A Mycophenolate Mofetil CEP of the European Pharmacopoeia monograph is often referred to as a Mycophenolate Mofetil Certificate of Suitability (COS). The purpose of a Mycophenolate Mofetil CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Mycophenolate Mofetil EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Mycophenolate Mofetil to their clients by showing that a Mycophenolate Mofetil CEP has been issued for it. The manufacturer submits a Mycophenolate Mofetil CEP (COS) as part of the market authorization procedure, and it takes on the role of a Mycophenolate Mofetil CEP holder for the record. Additionally, the data presented in the Mycophenolate Mofetil CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Mycophenolate Mofetil DMF.
A Mycophenolate Mofetil CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Mycophenolate Mofetil CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Mycophenolate Mofetil suppliers with CEP (COS) on PharmaCompass.
A Mycophenolate Mofetil written confirmation (Mycophenolate Mofetil WC) is an official document issued by a regulatory agency to a Mycophenolate Mofetil manufacturer, verifying that the manufacturing facility of a Mycophenolate Mofetil active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Mycophenolate Mofetil APIs or Mycophenolate Mofetil finished pharmaceutical products to another nation, regulatory agencies frequently require a Mycophenolate Mofetil WC (written confirmation) as part of the regulatory process.
click here to find a list of Mycophenolate Mofetil suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Mycophenolate Mofetil as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Mycophenolate Mofetil API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Mycophenolate Mofetil as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Mycophenolate Mofetil and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Mycophenolate Mofetil NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Mycophenolate Mofetil suppliers with NDC on PharmaCompass.
Mycophenolate Mofetil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Mycophenolate Mofetil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Mycophenolate Mofetil GMP manufacturer or Mycophenolate Mofetil GMP API supplier for your needs.
A Mycophenolate Mofetil CoA (Certificate of Analysis) is a formal document that attests to Mycophenolate Mofetil's compliance with Mycophenolate Mofetil specifications and serves as a tool for batch-level quality control.
Mycophenolate Mofetil CoA mostly includes findings from lab analyses of a specific batch. For each Mycophenolate Mofetil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Mycophenolate Mofetil may be tested according to a variety of international standards, such as European Pharmacopoeia (Mycophenolate Mofetil EP), Mycophenolate Mofetil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Mycophenolate Mofetil USP).
