
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
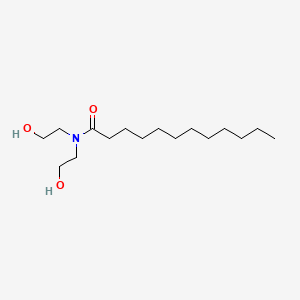

1. Lauramide Dea
2. Lauramide Diethanolamine
3. Lauric Acid Diethanolamide
1. 120-40-1
2. Lauric Diethanolamide
3. Lauryl Diethanolamide
4. Lauramide Dea
5. Lauroyl Diethanolamide
6. Lauric Acid Diethanolamide
7. Standamidd Ld
8. Alkamide Le
9. Dodecanamide, N,n-bis(2-hydroxyethyl)-
10. Rolamid Cd
11. Condensate Pl
12. Comperlan Ld
13. Mackamide Ll
14. Standamid Ld
15. Diethanollauramide
16. Hetamide Ml
17. Mackamide Llm
18. Richamide Std
19. Crillon Lde
20. Empilan Lde
21. Ethylan Mld
22. Rewomid Dlms
23. Stepan Lda
24. N,n-bis(2-hydroxyethyl)lauramide
25. Coco Diethanolamide
26. Varamid Ml 1
27. Varamide Ml 1
28. Lauroyldiethanolamine
29. Clindrol 100l
30. Clindrol 101cg
31. Clindrol 203cg
32. Clindrol 210cgn
33. Monamide 150lw
34. Richamide 6310
35. Super Amide L-9a
36. Super Amide L-9c
37. Synotol L-60
38. Unamide J-56
39. Aminon L 02
40. Onyxol 345
41. Bis(2-hydroxyethyl)lauramide
42. N,n-diethanollauramide
43. N,n-diethylollauramide
44. Rewomid Dl 203/s
45. Ninol P-621
46. Steinamid Dl 203 S
47. N,n-bis(hydroxyethyl)lauramide
48. Ninol 4821
49. Diethanolamine Lauroylamide
50. Clindrol Superamide 100l
51. Crillon L.d.e.
52. Lauric Acid Diethanolamine Condensate
53. N,n-diethanollauric Acid Amide
54. N,n-di(2-hydroxyethyl)lauramide
55. Emid 6511
56. Nci-c55323
57. Coconut Oil Amide Of Diethanolamine
58. N,n-bis(2-hydroxyethyl)laurylamide
59. N,n-bis(2-hydroxyethyl)lauroylamide
60. Amides, C12-22, N,n-bis(hydroxyethyl)
61. Lauric Acid Diethanolamine
62. I29i2vhg38
63. Lauric Acid Diethanolamine Condensate (1:1)
64. 72968-36-6
65. Lde
66. Ncgc00181001-01
67. Lankrostat Jp
68. Incromide Lr
69. Lauramido Dea
70. Lalmin D
71. Lda (surfactant)
72. Ninol Aa62
73. Clindrol 200l
74. Chemistat 2500
75. Ninol Aa-62 Extra
76. Chemstat Ld 100
77. Monamid 150-lw
78. Caswell No. 519a
79. Duspar La 2000
80. Ccris 4662
81. Hsdb 5586
82. N,n-bis(beta-hydroxyethyl)lauramide
83. Einecs 204-393-1
84. Epa Pesticide Chemical Code 079018
85. Methyl Laurate-diethanolamine Condensate
86. Brn 1791417
87. Unii-i29i2vhg38
88. Clindrol
89. Ai3-09484
90. Hartamide Lda
91. Stremid K
92. Ablumide Lde
93. Diethanol Lauramide
94. Mackamide L10
95. Schercomid Sl-ex
96. Carsamide Sal-7
97. Dehydat 10
98. Einecs 277-136-4
99. Lauric-diethanolamide
100. Alkamide 327
101. Schercomid Sl-extra
102. Witcamide 5138
103. Witcamide 5195
104. Clindrol 200 L
105. Lauric Acid Diethanolamine Condensation
106. Lauric Diethanol Amide
107. Monamid 150 Lw
108. Ninol Aa 62 Extra
109. (c12-22)-fatty Acids, Diethanolamide
110. N-lauroyldiethanolamine
111. Monamid 150-lmwc
112. Ninol 30-ll
113. Ninol Aa 62
114. Pionin D 1110
115. N,n-diethanoldodecanamide
116. N-dodecanoyldiethanolamine
117. Diethanolamide Lauric Acid
118. Dsstox_cid_5491
119. Diethanol Lauric Acid Amide
120. Ec 204-393-1
121. Lauric Acid-diethanol Amide
122. Dsstox_rid_77808
123. Dsstox_gsid_25491
124. Lauramide Dea [inci]
125. 4-04-00-01539 (beilstein Handbook Reference)
126. Mls006010225
127. Lauramide Dea [vandf]
128. Schembl284367
129. Schercomid 1214 (salt/mix)
130. Diethanolamide Of Methyl Laurate
131. Lauramide Dea [who-dd]
132. Chembl1996872
133. Dtxsid5025491
134. Emid 6541
135. Chebi:143726
136. Dtxsid101022608
137. Lauric Diethanolamide [ii]
138. Diethanol Lauramide [vandf]
139. Lauramide, N,n-bis(hydroxyethyl)-
140. Tox21_112653
141. Lmfa08040058
142. Mfcd00045982
143. Zinc38139837
144. Lauramide Diethanolamide (dea)
145. Akos014510507
146. Lauric Acid Diethanolamide, Aldrichcpr
147. Cs-w005513
148. N,n-bis(.beta.-hydroxyethyl)lauramide
149. As-10219
150. Cas-120-40-1
151. Smr001826330
152. Ft-0600339
153. Ft-0700556
154. N,n-di(2-hydroxyethyl)lauramide [hsdb]
155. A937450
156. Sr-01000944847
157. Sr-01000944847-1
158. W-109444
159. Q27280284
160. Z1591393781
161. N,n-bis(2-hydroxyethyl)dodecanamide, 9ci. N,n-bis(2-hydroxyethyl)lauramide
| Molecular Weight | 287.44 g/mol |
|---|---|
| Molecular Formula | C16H33NO3 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 14 |
| Exact Mass | 287.24604391 g/mol |
| Monoisotopic Mass | 287.24604391 g/mol |
| Topological Polar Surface Area | 60.8 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 216 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
EXPTL USE: NONIONIC SURFACE-ACTIVE AGENTS POSSESSING ETHER OR AMIDE LINKAGES BETWEEN HYDROPHILIC & HYDROPHOBIC PORTIONS OF THE MOLECULE RAPIDLY INACTIVATED INFECTIVITY OF HERPES SIMPLEX VIRUSES. ACTIVITY STEMMED FROM ABILITY OF NONIONIC SURFACTANTS TO DISSOLVE LIPID-CONTAINING MEMBRANES. PROPRIETARY VAGINAL CONTRACEPTIVE FORMULATIONS CONTAINING NONIONIC SURFACTANTS ALSO INACTIVATED HERPES SIMPLEX VIRUS INFECTIVITY. NONIONIC SURFACTANTS IN APPROPRIATE FORMULATION MAY EFFECTIVELY PREVENT HERPES SIMPLEX VIRUS TRANSMISSION.
ASCULAI SS ET AL; INACTIVATION OF HERPES SIMPLEX VIRUSES BY NONIONIC SURFACTANTS ANTIMICROB AGENTS CHEMOTHER 13(4) 686 (1978)
Market Place

ABOUT THIS PAGE
40
PharmaCompass offers a list of N,N-Diethanollauric Acid Amide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right N,N-Diethanollauric Acid Amide manufacturer or N,N-Diethanollauric Acid Amide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred N,N-Diethanollauric Acid Amide manufacturer or N,N-Diethanollauric Acid Amide supplier.
PharmaCompass also assists you with knowing the N,N-Diethanollauric Acid Amide API Price utilized in the formulation of products. N,N-Diethanollauric Acid Amide API Price is not always fixed or binding as the N,N-Diethanollauric Acid Amide Price is obtained through a variety of data sources. The N,N-Diethanollauric Acid Amide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A N,N-Diethanollauric Acid Amide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of N,N-Diethanollauric Acid Amide, including repackagers and relabelers. The FDA regulates N,N-Diethanollauric Acid Amide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. N,N-Diethanollauric Acid Amide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of N,N-Diethanollauric Acid Amide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A N,N-Diethanollauric Acid Amide supplier is an individual or a company that provides N,N-Diethanollauric Acid Amide active pharmaceutical ingredient (API) or N,N-Diethanollauric Acid Amide finished formulations upon request. The N,N-Diethanollauric Acid Amide suppliers may include N,N-Diethanollauric Acid Amide API manufacturers, exporters, distributors and traders.
click here to find a list of N,N-Diethanollauric Acid Amide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A N,N-Diethanollauric Acid Amide DMF (Drug Master File) is a document detailing the whole manufacturing process of N,N-Diethanollauric Acid Amide active pharmaceutical ingredient (API) in detail. Different forms of N,N-Diethanollauric Acid Amide DMFs exist exist since differing nations have different regulations, such as N,N-Diethanollauric Acid Amide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A N,N-Diethanollauric Acid Amide DMF submitted to regulatory agencies in the US is known as a USDMF. N,N-Diethanollauric Acid Amide USDMF includes data on N,N-Diethanollauric Acid Amide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The N,N-Diethanollauric Acid Amide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of N,N-Diethanollauric Acid Amide suppliers with USDMF on PharmaCompass.
N,N-Diethanollauric Acid Amide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of N,N-Diethanollauric Acid Amide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right N,N-Diethanollauric Acid Amide GMP manufacturer or N,N-Diethanollauric Acid Amide GMP API supplier for your needs.
A N,N-Diethanollauric Acid Amide CoA (Certificate of Analysis) is a formal document that attests to N,N-Diethanollauric Acid Amide's compliance with N,N-Diethanollauric Acid Amide specifications and serves as a tool for batch-level quality control.
N,N-Diethanollauric Acid Amide CoA mostly includes findings from lab analyses of a specific batch. For each N,N-Diethanollauric Acid Amide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
N,N-Diethanollauric Acid Amide may be tested according to a variety of international standards, such as European Pharmacopoeia (N,N-Diethanollauric Acid Amide EP), N,N-Diethanollauric Acid Amide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (N,N-Diethanollauric Acid Amide USP).
