
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 33069-62-4
2. P88xt4is4d
3. Taxol
4. Taxol A
5. Yewtaxan
6. Abraxane
7. Plaxicel
8. Genaxol
9. Ebetaxel
10. Genetaxyl
11. Capxol
12. Paxene
13. Onxol
14. Oncogel
15. Cyclopax
16. Genexol
17. Intaxel
18. Mitotax
19. Pacliex
20. Paxceed
21. Taxalbin
22. Empac
23. Abi-007
24. Onxal
25. Zisu
26. Lipopac
27. Taxus Liberte
28. Taxus Stent
29. Paclitaxel (taxol)
30. Endotag 1
31. Lep-etu
32. Genexol-pm
33. Tocosol Paclitaxel
34. Nsc-125973
35. (-)-paclitaxel
36. Taxus
37. Abi 007
38. Bms 181339-01
39. Mbt 0206
40. Nsc 125973
41. Bms-181339-01
42. Hsdb 6839
43. Dhp 107
44. Dhp-107
45. Drg-0190
46. Nk 105
47. Nsc125973
48. Qw 8184
49. Liposome-entrapped Paclitaxel Easy-to-use
50. Paclitaxel (taxus Canadensis)
51. Chembl428647
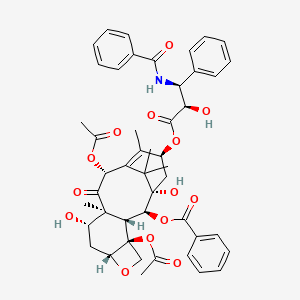
52. (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-9-(((2r,3s)-3-benzamido-2-hydroxy-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1h-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b-diyl Diacetate
53. 5beta,20-epoxy-1,2-alpha,4,7beta,10beta,13alpha-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester With (2r,3s)-n-benzoyl-3-phenylisoserine
54. Chebi:45863
55. Nab-paclitaxel
56. Mbt-0206
57. Abi-007 Component Paclitaxel
58. Nk-105
59. (2ar-(2aalpha,4beta,4abeta,6beta,9alpha(alpha R*,betas*),11alpha,12alpha,12balpha))-beta-(benzoylamino)-alpha-hydroxybenzenepropanoic Acid 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h-cyclodeca(3,4)benz(1,2-b)oxet-9-yl Ester
60. Qw-8184
61. Ncgc00164367-01
62. Padexol
63. Mfcd00869953
64. Cynviloq
65. Nanoxel
66. Sindaxel
67. Xorane
68. Cypher Select
69. Coroflex Please
70. Taxus Express
71. 7,11-methano-1h-cyclodeca[3,4]benz[1,2-b]oxete, Benzenepropanoic Acid Deriv.
72. (1s,2s,3r,4s,7r,9s,10s,12r,15s)-4,12-bis(acetyloxy)-1,9-dihydroxy-15-{[(2r,3s)-2-hydroxy-3-phenyl-3-(phenylformamido)propanoyl]oxy}-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0^{3,10}.0^{4,7}]heptadec-13-en-2-yl Benzoate
73. (nab)-paclitaxel
74. [(1s,2s,3r,4s,7r,9s,10s,12r,15s)-4,12-diacetyloxy-15-[(2r,3s)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] Benzoate
75. Smr000857385
76. Endotag-1
77. Sr-01000075350
78. Abraxane I.v. Suspension
79. Unii-p88xt4is4d
80. Anzatax
81. Infinnium
82. Nanotaxel
83. Paclical
84. Pacligel
85. Paxoral
86. Ccris 8143
87. Paclitaxel,(s)
88. Paclitaxel [usan:usp:inn:ban]
89. Abraxane (tn)
90. (2alpha,5beta,7beta,10beta,13alpha)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-({(2r,3s)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-9-oxo-5,20-epoxytax-11-en-2-yl Benzoate
91. [diacetoxy-[(2r,3s)-3-benzamido-2-hydroxy-3-phenyl-propanoyl]oxy-dihydroxy-tetramethyl-oxo-[?]yl] Benzoate
92. 5?,20-epoxy-1,7?-dihydroxy-9-oxotax-11-ene-2?,4,10?,13?-tetrayl 4,10-diacetate 2-benzoate 13-[(2r,3s)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate]
93. Nanoparticle Albumin Bound Paclitaxel
94. Cas-33069-62-4
95. Ig 001
96. Bms-181339
97. Paclitaxel-ssmm-vip
98. P-ssmm-vip
99. Paclitaxel [mi]
100. Paclitaxel [inn]
101. Paclitaxel [jan]
102. Prestwick3_000155
103. Paclitaxel [hsdb]
104. Paclitaxel [usan]
105. Dsstox_cid_3413
106. Taxol (tn)
107. Paclitaxel [vandf]
108. Paclitaxel [mart.]
109. Schembl3976
110. Dsstox_rid_77016
111. Nova-12005
112. Paclitaxel [usp-rs]
113. Paclitaxel [who-dd]
114. Paclitaxel, Taxus Brevifolia
115. Bidd:pxr0046
116. Dsstox_gsid_23413
117. Bspbio_000290
118. Kbiogr_002509
119. Kbioss_002517
120. Paclitaxel (jan/usp/inn)
121. Mls002154218
122. Mls002695976
123. Oas-pac-100
124. Paclitaxel [ema Epar]
125. Bpbio1_000320
126. Gtpl2770
127. Megxp0_001940
128. Taxol (tn) (bristol Meyers)
129. Paclitaxel [green Book]
130. Dtxsid9023413
131. Paclitaxel [orange Book]
132. Tsd-001 [who-dd]
133. Acon1_002231
134. Kbio2_002509
135. Kbio2_005077
136. Kbio2_007645
137. Kbio3_002987
138. Anx-513
139. Dhp-208
140. Dts-301
141. Paclitaxel [ep Monograph]
142. Paclitaxel [usp Impurity]
143. Sdp-013
144. Cmap_000068
145. Oraxol Component Paclitaxel
146. Hms2090d07
147. Hms2095o12
148. Hms2231a16
149. Hms3712o12
150. Paclitaxel [usp Monograph]
151. Abraxane (albumin-bound Suspension)
152. Abraxane Component Paclitaxel
153. Act02709
154. Hy-b0015
155. Mpi-5018
156. Tox21_112107
157. Bdbm50001839
158. Nsc745099
159. Zinc96006020
160. Abi 007 Component Paclitaxel
161. Akos007930675
162. Akos015969673
163. Akos025312303
164. Ccg-220155
165. Cs-1145
166. Db01229
167. Gs-6554
168. Nsc-745099
169. Ncgc00164367-02
170. Ncgc00164367-03
171. Ncgc00164367-04
172. Ncgc00164367-05
173. Ncgc00164367-10
174. Paclitaxel, From Taxus Brevifolia, 95%
175. (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5h-cyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester With (2r,3s)-n-benzoyl-3-phenylisoserine
176. Nab-paclitaxel Component Paclitaxel
177. Nci60_000601
178. Paclitaxel, From Taxus Yannanensis, Powder
179. Ab00513812
180. N1361
181. D00491
182. M02242
183. N88686
184. Ab00513812-02
185. Ab00513812-03
186. 069p624
187. Paclitaxel, Antibiotic For Culture Media Use Only
188. Q423762
189. 7,4]benz[1,2-b]oxete,benzenepropanoic Acid Deriv.
190. Q-201533
191. Sr-01000075350-1
192. Sr-01000075350-3
193. Sr-01000075350-6
194. Sr-01000075350-7
195. Sr-01000075350-9
196. Brd-k62008436-001-03-1
197. Brd-k62008436-001-05-6
198. Brd-k62008436-001-22-1
199. Paclitaxel, From Semisynthetic (from Taxus Sp.), >=97%
200. Paclitaxel, European Pharmacopoeia (ep) Reference Standard
201. Paclitaxel, From Taxus Brevifolia, >=95% (hplc), Powder
202. Paclitaxel, United States Pharmacopeia (usp) Reference Standard
203. 12-benzoate, 9-ester With (2r,3s)-n-benzoyl-3-phenylisoserine
204. Paclitaxel, Pharmaceutical Secondary Standard; Certified Reference Material
205. Paclitaxel Natural For Peak Identification, European Pharmacopoeia (ep) Reference Standard
206. (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro 4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano 5hcyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate,
207. (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h-cyclodeca[3,4]benz[1,2-b]oxet-9-yl (ar,bs)-b-(benzoylamino)-a-hydroxybenzenepropanoate
208. (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-9-(((2r,3s)-3-benzamido-2-hydroxy-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1h-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2ah)-diyl Diacetate
209. (2ar-(2aalpha,4beta,4abeta,6beta,9alpha(alpha R*,betas*),11alpha,12alpha,12balpha))-beta-(benzoylamino)-alpha-hydroxybenzenepropanoic Acid 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12
210. (2beta,5beta,7alpha,8alpha,10alpha,13alpha)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-({(2r,3s)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-9-oxo-5,20-epoxytax-11-en-2-yl Benzoate
211. 1203669-79-7
212. 4alpha,10beta-bis(acetyloxy)-13alpha-[(2s,3s)-3-benzamido-2-hydroxy-3-phenylpropanoyloxy]-1,7beta-dihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl Benzoate
213. Benzenepropanoic Acid, .beta.-(benzoylamino)-.alpha.-hydroxy-, (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h-cyclodeca(3,4)benz(1,2-b)oxet-9-yl Ester, (.alpha.r,.beta.s)-
214. Benzenepropanoic Acid, 6,12b-bis(acetyl Oxy)-12-(benzoyloxy)- 2a,3,4,4a,5,6,9,10,11,12,12a,12b,- Dodecahydro-4,11- Dihydroxy-4a,8,13,13-tetramethyl-5-oxo- 7,11-methano- 1h-cyclodeca[3,4]benz[1,2-b]oxet-9-yl Ester, [2ar- [2a.alpha.,4.beta.,4a.beta.,6.beta.,9.alpha.(alpha. R*,.beta.s*),11.alpha.,12.alpha.,12a.alpha.,12b.alpha.]]-
215. Benzenepropanoic Acid, B-(benzoylamino)-.alpha.-hydroxy-, (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h-cyclodeca[3,4]benz[1,2-b]oxet-9-yl Ester, (ar,bs)-
216. Benzenepropanoic Acid, Beta-(benzoylamino)-alpha-hydroxy-, (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13
217. Benzenepropanoic Acid, Beta-(benzoylamino)-alpha-hydroxy-, (2ar,4s,4as,6r,9s,11s,12s,12ar,12bs)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h-cyclodeca(3,4)benz(1,2-b)oxet-9-yl Ester, (alphar,betas)-
218. Benzenepropanoic Acid, Beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h
219. Benzenepropanoic Acid, Beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h-cyclodeca(3,4)benz(1,2-b)oxet-9-yl Ester, (2ar-(2a-alpha,4-beta,4a-beta,6-beta,9-alpha(alpha-r*,beta-s*),11-alpha,12-alpha,12a-alpha, 12b-alpha))-
220. Benzenepropanoic Acid, Beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1h-cyclodeca(3,4)benz(1,2-b)oxet-9-yl Ester, (2ar-(2aalpha,4beta,4abeta,6beta,9alpha(alphar*,betas*),11alpha,12alpha,12aalpha,12balpha))-
221. Paclitaxel Semi-synthetic For Peak Identification, European Pharmacopoeia (ep) Reference Standard
222. Paclitaxel Semi-synthetic For System Suitability, European Pharmacopoeia (ep) Reference Standard
223. Tax-11-en-9-one, 5beta,20-epoxy-1,2alpha,4,7beta,10beta,13alpha- Hexahydroxy-, 4,10-diacetate 2-benzoate, 13-ester With (2r,3s)-n-benzoyl-3-phenylisoserine
224. Tax-11-en-9-one, 5beta,20-epoxy-1,2alpha,4,7beta,10beta,13alpha-hexahydroxy-, 4,10-diacetate 2-benzoate 13-ester With (2r,3s)-n-benzoyl-3-phenylisoserine (8ci)
225. Tax-11-en-9-one,20-epoxy-1,2.alpha.,4,7.beta., 10.beta.,13.alpha.- Hexahydroxy-, 4,10-diacetate 2- Benzoate,13-ester With (2r,3s)-n-benzoyl-3-phenylisoserine
| Molecular Weight | 853.9 g/mol |
|---|---|
| Molecular Formula | C47H51NO14 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 14 |
| Exact Mass | 853.33095530 g/mol |
| Monoisotopic Mass | 853.33095530 g/mol |
| Topological Polar Surface Area | 221 Ų |
| Heavy Atom Count | 62 |
| Formal Charge | 0 |
| Complexity | 1790 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 11 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Abraxane |
| PubMed Health | Paclitaxel Protein-bound (Injection) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Paclitaxel |
| Dosage Form | For suspension |
| Route | Iv (infusion) |
| Strength | 100mg/vial |
| Market Status | Prescription |
| Company | Abraxis Bioscience |
| 2 of 6 | |
|---|---|
| Drug Name | Paclitaxel |
| PubMed Health | Paclitaxel (Injection) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Paclitaxel |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6mg/ml |
| Market Status | Prescription |
| Company | Hospira; Fresenius Kabi Oncol; Actavis Elizabeth; Teva Pharms; Ebewe Pharma; Onco Therapies; Eurohlth Intl |
| 3 of 6 | |
|---|---|
| Drug Name | Taxol |
| PubMed Health | Paclitaxel (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Paclitaxel Injection, USP is a clear colorless to slightly yellow viscous solution. It is supplied as a nonaqueous solution intended for dilution with a suitable parenteral fluid prior to intravenous infusion. Paclitaxel Injection, USP is available i... |
| Active Ingredient | Paclitaxel |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6mg/ml |
| Market Status | Prescription |
| Company | Hq Spclt Pharma |
| 4 of 6 | |
|---|---|
| Drug Name | Abraxane |
| PubMed Health | Paclitaxel Protein-bound (Injection) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Paclitaxel |
| Dosage Form | For suspension |
| Route | Iv (infusion) |
| Strength | 100mg/vial |
| Market Status | Prescription |
| Company | Abraxis Bioscience |
| 5 of 6 | |
|---|---|
| Drug Name | Paclitaxel |
| PubMed Health | Paclitaxel (Injection) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Paclitaxel |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6mg/ml |
| Market Status | Prescription |
| Company | Hospira; Fresenius Kabi Oncol; Actavis Elizabeth; Teva Pharms; Ebewe Pharma; Onco Therapies; Eurohlth Intl |
| 6 of 6 | |
|---|---|
| Drug Name | Taxol |
| PubMed Health | Paclitaxel (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Paclitaxel Injection, USP is a clear colorless to slightly yellow viscous solution. It is supplied as a nonaqueous solution intended for dilution with a suitable parenteral fluid prior to intravenous infusion. Paclitaxel Injection, USP is available i... |
| Active Ingredient | Paclitaxel |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6mg/ml |
| Market Status | Prescription |
| Company | Hq Spclt Pharma |
Antineoplastic Agents, Phytogenic; Radiation-Sensitizing Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Standard formulation paclitaxel requires the use of solvents, such as Cremphor-EL, which contribute to some of the toxicities commonly associated with paclitaxel-based therapy. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a novel solvent-free formulation of paclitaxel. The formulation is prepared by high-pressure homogenization of paclitaxel in the presence of serum albumin into a nanoparticle colloidal suspension. The human albumin-stabilized paclitaxel particles have an average size of 130 nm. Nab-paclitaxel has several practical advantages over Cremphor-EL-paclitaxel, including a shorter infusion time (30 min) and no need for premedications for hypersensitivity reactions. The nab-paclitaxel formulation eliminates the impact of Cremphor-EL on paclitaxel pharmacokinetics and utilizes the endogenous albumin transport mechanisms to concentrate nab-paclitaxel within the tumor. A recent Phase III trial compared nab- and Cremphor-EL-paclitaxel in patients with metastatic breast cancer. Patients treated with nab-paclitaxel experienced a higher response, longer time to tumor progression and, in patients receiving second-line or greater therapy, a longer median survival. Patients treated with nab-paclitaxel had a significantly lower rate of severe neutropenia and a higher rate of sensory neuropathy. The preclinical and clinical data indicate that the nab-paclitaxel formulation has significant advantages over Cremphor-EL-paclitaxel.
PMID:17716129 Stinchcombe TE; Nanomed 2 (4): 415-23 (2007)
As first line and subsequent therapy for the treatment of advanced carcinoma of the ovary. As first-line therapy, paclitaxel is indicated in combination with cisplatin.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 2833
Adjuvant treatment of node-positive breast cancer administration sequentially to standard doxorubicin-containing combination chemotherapy. In the clinical trial, there was an overall favorable effect on disease-free and overall survival in the total population of patients with receptor-positive and receptor-negative tumors, but the benefit has been specifically demonstrated by available data (mean follow-up, 30 months) only in the patients with estrogen and progesterone receptor-negative tumors. Indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Previous therapy should have included an anthracycline unless clinically contraindicated.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 2833
For more Therapeutic Uses (Complete) data for TAXOL (9 total), please visit the HSDB record page.
Administer paclitaxel under the supervision of a health care provider experienced in the use of cancer chemotherapeutic agents. Appropriate management of complications is possible only when adequate diagnostic and treatment facilitates are readily available.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 2833
Do not give paclitaxel therapy to patients with solid tumors who have baseline neutrophil counts of less than 1,500 cells/cu mm, and do not give to patients with AIDS-related Kaposi sarcoma if the baseline neutrophil count is less than 1,000 cells/cu mm. In order to monitor the occurrence of bone marrow suppression, primarily neutropenia, which may be severe and result in infection, perform frequent peripheral blood cell counts on all patients receiving paclitaxel.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 2833
Anaphylaxis and severe hypersensitivity reactions characterized by dyspnea and hypotension requiring treatment, angioedema, and generalized urticaria have occurred in 2% to 4% of patients receiving paclitaxel in clinical trials. Fatal reactions have occurred in patients despite premedication. Pretreat all patients with corticosteroids, diphenhydramine, and H2 antagonists. Do not rechallenge patients who experience severe hypersensitivity reactions to paclitaxel with the drug.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 2833
An albumin form of paclitaxel may substantially affect a drug's functional properties relative to those of drug in solution. Do not substitute for or with other paclitaxel formulations. /Paclitaxel (albumin-bound)/
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 2833
For more Drug Warnings (Complete) data for TAXOL (62 total), please visit the HSDB record page.
Used in the treatment of Kaposi's sarcoma and cancer of the lung, ovarian, and breast. Abraxane is specfically indicated for the treatment of metastatic breast cancer and locally advanced or metastatic non-small cell lung cancer.
FDA Label
Pazenir monotherapy is indicated for the treatment of metastatic breast cancer in adult patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline containing therapy is not indicated.
Pazenir in combination with carboplatin is indicated for the first-line treatment of non-small cell lung cancer in adult patients who are not candidates for potentially curative surgery and/or radiation therapy.
Abraxane monotherapy is indicated for the treatment of metastatic breast cancer in adult patients who have failed first-line treatment for metastatic disease and for whom standard, anthracycline containing therapy is not indicated.
Abraxane in combination with gemcitabine is indicated for the first-line treatment of adult patients with metastatic adenocarcinoma of the pancreas.
Abraxane in combination with carboplatin is indicated for the first-line treatment of non-small cell lung cancer in adult patients who are not candidates for potentially curative surgery and/or radiation therapy.
Apealea in combination with carboplatin is indicated for the treatment of adult patients with first relapse of platinumsensitive epithelial ovarian cancer , primary peritoneal cancer and fallopian tube cancer .
Paxene is indicated for the treatment of patients with:
advanced AIDS-related Kaposi's sarcoma (AIDS-KS) who have failed prior liposomal anthracycline therapy;
metastatic carcinoma of the breast (MBC) who have failed, or are not candidates for standard anthracycline-containing therapy;
advanced carcinoma of the ovary (AOC) or with residual disease (> 1 cm) after initial laparotomy, in combination with cisplatin as first-line treatment;
metastatic carcinoma of the ovary (MOC) after failure of platinum-containing combination therapy without taxanes as second-line treatment;
non-small cell lung carcinoma (NSCLC) who are not candidates for potentially curative surgery and/or radiation therapy, in combination with cisplatin. Limited efficacy data supports this indication (see section 5. 1).
Treatment of solid malignant tumours
Treatment of soft tissue sarcoma
Paclitaxel is a taxoid antineoplastic agent indicated as first-line and subsequent therapy for the treatment of advanced carcinoma of the ovary, and other various cancers including breast cancer. Paclitaxel is a novel antimicrotubule agent that promotes the assembly of microtubules from tubulin dimers and stabilizes microtubules by preventing depolymerization. This stability results in the inhibition of the normal dynamic reorganization of the microtubule network that is essential for vital interphase and mitotic cellular functions. In addition, paclitaxel induces abnormal arrays or "bundles" of microtubules throughout the cell cycle and multiple asters of microtubules during mitosis.
L01CD01
L01CD01
L01CD01
L01CD01
L01CD01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01C - Plant alkaloids and other natural products
L01CD - Taxanes
L01CD01 - Paclitaxel
Absorption
When a 24 hour infusion of 135 mg/m^2 is given to ovarian cancer patients, the maximum plasma concentration (Cmax) is 195 ng/mL, while the AUC is 6300 ngh/mL.
Route of Elimination
In 5 patients administered a 225 or 250 mg/m2 dose of radiolabeled paclitaxel as a 3-hour infusion, a mean of 71% of the radioactivity was excreted in the feces in 120 hours, and 14% was recovered in the urine.
Volume of Distribution
227 to 688 L/m^2 [apparent volume of distribution at steady-state, 24 hour infusion]
Clearance
21.7 L/h/m2 [Dose 135 mg/m2, infusion duration 24 h]
23.8 L/h/m2 [Dose 175 mg/m2, infusion duration 24 h]
7 L/h/m2 [Dose 135 mg/m2, infusion duration 3 h]
12.2 L/h/m2 [Dose 175 mg/m2, infusion duration 3 h]
Paclitaxel bound to nanoparticles of the serum protein albumin is delivered via endothelial transport mediated by albumin receptors, and the resulting concentration of paclitaxel in tumor cells is increased compared with that achieved using an equivalent dose of conventional paclitaxel. Like conventional paclitaxel, albumin-bound paclitaxel has a large volume of distribution. Following 30-minute or 3-hour IV infusion of 80-375 mg/sq m albumin-bound paclitaxel, the volume of distribution averaged 632 L/sq m. The volume of distribution of albumin-bound paclitaxel 260 mg/sq m by 30-minute IV infusion was 53% larger than the volume of distribution of conventional paclitaxel 175 mg/sq m by 3-hour IV infusion. /Paclitaxel (albumin-bound)/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1188
Following IV administration, paclitaxel is widely distributed into body fluids and tissues. Paclitaxel has a large volume of distribution that appears to be affected by dose and duration of infusion. Following administration of paclitaxel doses of 135 or 175 mg/sq m by IV infusion over 24 hours in patients with advanced ovarian cancer, the mean apparent volume of distribution at steady state ranged from 227-688 L/sq m. The steady-state volume of distribution ranged from 18.9-260 L/sq m in children with solid tumors or refractory leukemia receiving paclitaxel 200-500 mg/sq m by 24-hour IV infusion. Paclitaxel does not appear to readily penetrate the CNS, but paclitaxel has been detected in ascitic fluid following IV infusion of the drug. It is not known whether paclitaxel is distributed into human milk, but in lactating rats given radiolabeled paclitaxel, concentrations of radioactivity in milk were higher than those in plasma and declined in parallel with plasma concentrations of the drug.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1188
For the dose range 80-375 mg/sq m, increase in dose of albumin-bound paclitaxel was associated with a proportional increase in AUC.354 The duration of infusion did not affect the pharmacokinetic disposition of albumin-bound paclitaxel. Following 30-minute or 3-hour IV infusion of albumin-bound paclitaxel 260 mg/sq m, the peak plasma concentration averaged 18,741 ng/mL. /Paclitaxel (albumin-bound)/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1188
Peak plasma concentrations and areas under the plasma concentration-time curve (AUCs) following IV administration of paclitaxel exhibit marked interindividual variation. Plasma concentrations of paclitaxel increase during continuous IV administration of the drug and decline immediately following completion of the infusion. Following 24-hour IV infusion of paclitaxel at doses of 135 or 175 mg/sq m in patients with advanced ovarian cancer, peak plasma concentrations averaged 195 or 365 ng/mL, respectively; the increase in dose (30%) was associated with a disproportionately greater increase in peak plasma concentration (87%), but the increase in AUC was proportional. When paclitaxel was administered by continuous IV infusion over 3 hours at doses of 135 or 175 mg/sq m in patients with advanced ovarian cancer, peak plasma concentrations averaged 2.17 or 3.65 ug/mL, respectively; the increase in dose (30%) was associated with disproportionately greater increases in peak plasma concentration (68%) and AUC (89%).
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1188
For more Absorption, Distribution and Excretion (Complete) data for TAXOL (8 total), please visit the HSDB record page.
Hepatic. In vitro studies with human liver microsomes and tissue slices showed that paclitaxel was metabolized primarily to 6a-hydrox-ypaclitaxel by the cytochrome P450 isozyme CYP2C8; and to two minor metabolites, 3’-p-hydroxypaclitaxel and 6a, 3’-p-dihydroxypaclitaxel, by CYP3A4.
Paclitaxel is extensively metabolized in the liver. Metabolism of paclitaxel to its major metabolite, 6alpha-hydroxypaclitaxel, is mediated by cytochrome P-450 isoenzyme CYP2C8,1 185 187 202 354 while metabolism to 2 of its minor metabolites, 3'-p-hydroxypaclitaxel and 6alpha,3'-p-dihydroxypaclitaxel, is catalyzed by CYP3A4.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1188
The elimination of nonradioactive taxol in bile and urine was investigated in the rat after administration via the caudal vein (10 mg/kg). As in humans, no metabolites of taxol were detected by HPLC in rat urine, and only 10% of the injected taxol was recovered in urine over a 24 hr period. In contrast, 11.5% and 29% of the injected taxol was recovered in rat bile as unchanged taxol and metabolites, respectively. Among the nine taxol metabolites detected by HPLC, the side chain at C13, which is required for pharmacological activity, had been removed in only one minor metabolite, baccatin III. The chemical structures of the two major hydroxylated metabolites were determined by MS (fast atom bombardment and desorption chemical ionization) and (1)H NMR spectroscopy. One was a taxol derivative hydroxylated on the phenyl group at C3 of the side chain at C13, while the other corresponded to a taxol derivative hydroxylated in the m-position on the benzoate of the side chain at C2. Although these two major taxol metabolites were as active as taxol in preventing cold microtubule disassembly, they were, respectively, 9 and 39 times less cytotoxic as taxol on in vitro L1210 leukemia growth. These results show for the first time that there is a significant hepatic metabolism of taxol.
PMID:1981534 Monsarrat B et al; Drug Metab Dispos Biol Fate Chem 18 (6): 895-901 (1990)
To investigate how taxane's substituents at C3' affect its metabolism, ... the metabolism of cephalomannine and paclitaxel, a pair of analogs that differ slightly at the C3' position /was compared/. After cephalomannine was incubated with human liver microsomes in an NADPH-generating system, two monohydroxylated metabolites (M1 and M2) were detected by liquid chromatography/tandem mass spectrometry. C4'' (M1) and C6alpha (M2) were proposed as the possible hydroxylation sites, and the structure of M1 was confirmed by (1)H NMR. Chemical inhibition studies and assays with recombinant human cytochromes P450 (P450s) indicated that 4''-hydroxycephalomannine was generated predominantly by CYP3A4 and 6alpha-hydroxycephalomannine by CYP2C8. The overall biotransformation rate between paclitaxel and cephalomannine differed slightly (184 vs. 145 pmol/min/mg), but the average ratio of metabolites hydroxylated at the C13 side chain to C6alpha for paclitaxel and cephalomannine varied significantly (15:85 vs. 64:36) in five human liver samples. Compared with paclitaxel, the major hydroxylation site transferred from C6alpha to C4'', and the main metabolizing P450 changed from CYP2C8 to CYP3A4 for cephalomannine. In the incubation system with rat or minipig liver microsomes, only 4''-hydroxycephalomannine was detected, and its formation was inhibited by CYP3A inhibitors. Molecular docking by AutoDock suggested that cephalomannine adopted an orientation in favor of 4''-hydroxylation, whereas paclitaxel adopted an orientation favoring 3'-p-hydroxylation. Kinetic studies showed that CYP3A4 catalyzed cephalomannine more efficiently than paclitaxel due to an increased V(m). Our results demonstrate that relatively minor modification of taxane at C3' has major consequence on the metabolism.
PMID:18039807 Zhang JW et al; Drug Metab Dispos 36 (2): 418-26 (2008)
When a 24 hour infusion of 135 mg/m^2 is given to ovarian cancer patients, the elimination half=life is 52.7 hours.
5.3-17.4 hours after 1 and 6 hour infusions at dosing levels of 15-275 mg/sq m
Lelkin, J.B., Paloucek, F.P., Poisoning & Toxicology Compendium. LEXI-COMP Inc. & American Pharmaceutical Association, Hudson, OH 1998., p. 433
Following IV infusion of paclitaxel over periods ranging from 6-24 hours in adults with malignancy, plasma concentrations of paclitaxel appeared to decline in a biphasic manner in some studies, with an average distribution half-life of 0.34 hours and an average elimination half-life of 5.8 hours. However, additional studies, particularly those in which paclitaxel is administered over shorter periods of infusion, show that the drug exhibits nonlinear pharmacokinetic behavior. In patients receiving paclitaxel 175 mg/sq m administered by 3-hour IV infusion, the distribution half-life averages 0.27 hours and the elimination half-life averages 2.33 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1188
Following 30-minute or 3-hour IV infusion of 80-375 mg/sq m albumin-bound paclitaxel, ... terminal half-life albumin-bound paclitaxel was about 27 hours. ... /Paclitaxel (albumin-bound)/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1188
Paclitaxel interferes with the normal function of microtubule growth. Whereas drugs like colchicine cause the depolymerization of microtubules in vivo, paclitaxel arrests their function by having the opposite effect; it hyper-stabilizes their structure. This destroys the cell's ability to use its cytoskeleton in a flexible manner. Specifically, paclitaxel binds to the β subunit of tubulin. Tubulin is the "building block" of mictotubules, and the binding of paclitaxel locks these building blocks in place. The resulting microtubule/paclitaxel complex does not have the ability to disassemble. This adversely affects cell function because the shortening and lengthening of microtubules (termed dynamic instability) is necessary for their function as a transportation highway for the cell. Chromosomes, for example, rely upon this property of microtubules during mitosis. Further research has indicated that paclitaxel induces programmed cell death (apoptosis) in cancer cells by binding to an apoptosis stopping protein called Bcl-2 (B-cell leukemia 2) and thus arresting its function.
Evidence suggests that paclitaxel also may induce cell death by triggering apoptosis. In addition, paclitaxel and docetaxel enhance the effects of ionizing radiation, possibly by blocking cells in the G2 phase, the phase of the cell cycle in which cells are most radiosensitive.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1187
Paclitaxel is an antimicrotubule antineoplastic agent. Unlike some other common antimicrotubule agents (e.g., vinca alkaloids, colchicine, podophyllotoxin), which inhibit microtubule assembly, paclitaxel and docetaxel (a semisynthetic taxoid) promote microtubule assembly. Microtubules are organelles that exist in a state of dynamic equilibrium with their components, tubulin dimers. They are an essential part of the mitotic spindle and also are involved in maintenance of cell shape and motility, and transport between organelles within the cell. By binding in a reversible, concentration-dependent manner to the beta-subunit of tubulin at the N-terminal domain, paclitaxel enhances the polymerization of tubulin, the protein subunit of the spindle microtubules, even in the absence of factors that are normally required for microtubule assembly (e.g., guanosine triphosphate [GTP]), and induces the formation of stable, nonfunctional microtubules. Paclitaxel promotes microtubule stability even under conditions that typically cause depolymerization in vitro (e.g., cold temperature, the addition of calcium, the presence of antimitotic drugs). While the precise mechanism of action of the drug is not understood fully, paclitaxel disrupts the dynamic equilibrium within the microtubule system and blocks cells in the late G2 phase and M phase of the cell cycle, inhibiting cell replication.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1187
... Taxol induces tubulin polymerization and forms extremely stable and nonfunctional microtubules. Taxol has demonstrated broad activity in preclinical screening studies, and antineoplastic activity has been observed in several classically refractory tumors. These tumors include cisplatin resistant ovarian carcinoma in phase II trials and malignant melanoma and non-small cell lung carcinoma in phase I studies.
PMID:1973737 Rowinsky EK et al; J Natl Cancer Inst 82 (15): 1247-59 (1990)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
91
PharmaCompass offers a list of Paclitaxel API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Paclitaxel manufacturer or Paclitaxel supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Paclitaxel manufacturer or Paclitaxel supplier.
PharmaCompass also assists you with knowing the Paclitaxel API Price utilized in the formulation of products. Paclitaxel API Price is not always fixed or binding as the Paclitaxel Price is obtained through a variety of data sources. The Paclitaxel Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nab-paclitaxel manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nab-paclitaxel, including repackagers and relabelers. The FDA regulates Nab-paclitaxel manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nab-paclitaxel API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nab-paclitaxel manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nab-paclitaxel supplier is an individual or a company that provides Nab-paclitaxel active pharmaceutical ingredient (API) or Nab-paclitaxel finished formulations upon request. The Nab-paclitaxel suppliers may include Nab-paclitaxel API manufacturers, exporters, distributors and traders.
click here to find a list of Nab-paclitaxel suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nab-paclitaxel DMF (Drug Master File) is a document detailing the whole manufacturing process of Nab-paclitaxel active pharmaceutical ingredient (API) in detail. Different forms of Nab-paclitaxel DMFs exist exist since differing nations have different regulations, such as Nab-paclitaxel USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nab-paclitaxel DMF submitted to regulatory agencies in the US is known as a USDMF. Nab-paclitaxel USDMF includes data on Nab-paclitaxel's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nab-paclitaxel USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nab-paclitaxel suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nab-paclitaxel Drug Master File in Japan (Nab-paclitaxel JDMF) empowers Nab-paclitaxel API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nab-paclitaxel JDMF during the approval evaluation for pharmaceutical products. At the time of Nab-paclitaxel JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nab-paclitaxel suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Nab-paclitaxel Drug Master File in Korea (Nab-paclitaxel KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Nab-paclitaxel. The MFDS reviews the Nab-paclitaxel KDMF as part of the drug registration process and uses the information provided in the Nab-paclitaxel KDMF to evaluate the safety and efficacy of the drug.
After submitting a Nab-paclitaxel KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Nab-paclitaxel API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Nab-paclitaxel suppliers with KDMF on PharmaCompass.
A Nab-paclitaxel CEP of the European Pharmacopoeia monograph is often referred to as a Nab-paclitaxel Certificate of Suitability (COS). The purpose of a Nab-paclitaxel CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Nab-paclitaxel EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Nab-paclitaxel to their clients by showing that a Nab-paclitaxel CEP has been issued for it. The manufacturer submits a Nab-paclitaxel CEP (COS) as part of the market authorization procedure, and it takes on the role of a Nab-paclitaxel CEP holder for the record. Additionally, the data presented in the Nab-paclitaxel CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Nab-paclitaxel DMF.
A Nab-paclitaxel CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Nab-paclitaxel CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Nab-paclitaxel suppliers with CEP (COS) on PharmaCompass.
A Nab-paclitaxel written confirmation (Nab-paclitaxel WC) is an official document issued by a regulatory agency to a Nab-paclitaxel manufacturer, verifying that the manufacturing facility of a Nab-paclitaxel active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Nab-paclitaxel APIs or Nab-paclitaxel finished pharmaceutical products to another nation, regulatory agencies frequently require a Nab-paclitaxel WC (written confirmation) as part of the regulatory process.
click here to find a list of Nab-paclitaxel suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nab-paclitaxel as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nab-paclitaxel API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nab-paclitaxel as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nab-paclitaxel and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nab-paclitaxel NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nab-paclitaxel suppliers with NDC on PharmaCompass.
Nab-paclitaxel Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nab-paclitaxel GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nab-paclitaxel GMP manufacturer or Nab-paclitaxel GMP API supplier for your needs.
A Nab-paclitaxel CoA (Certificate of Analysis) is a formal document that attests to Nab-paclitaxel's compliance with Nab-paclitaxel specifications and serves as a tool for batch-level quality control.
Nab-paclitaxel CoA mostly includes findings from lab analyses of a specific batch. For each Nab-paclitaxel CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nab-paclitaxel may be tested according to a variety of international standards, such as European Pharmacopoeia (Nab-paclitaxel EP), Nab-paclitaxel JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nab-paclitaxel USP).
