
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
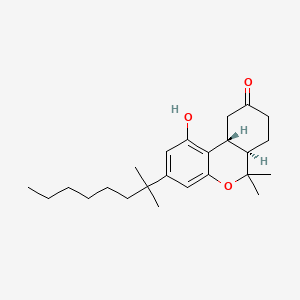

1. Cesamet
2. Lilly 109514
3. Ly 109514
4. Nabilone, (6ar-trans)-isomer
5. Nabilone, (6as-trans)-isomer
6. Nabilone, (cis-(+-))-isomer
7. Nabilone, (trans-(+-))-isomer
1. 51022-71-0
2. Cesamet
3. Chembl947
4. Lilly 109514
5. 2n4o9l084n
6. Cpd 109514
7. Cpd-109514
8. (6ar,10ar)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)-7,8,10,10a-tetrahydro-6ah-benzo[c]chromen-9-one
9. Nabilona
10. Nabilonum
11. Nabilonum [latin]
12. Nabilona [spanish]
13. Nabilone [usan:inn:ban]
14. Unii-2n4o9l084n
15. 9h-dibenzo(b,d)pyran-9-one, 3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-, (6ar,10ar)-rel-
16. 9h-dibenzo[b,d]pyran-9-one, 3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-, (6ar,10ar)-rel-
17. Cesamet (tn)
18. (-)-nabilone
19. (?)-nabilone
20. Nabilone [usan]
21. Nabilone (usan/inn)
22. Nabilone [inn]
23. Nabilone [mi]
24. Nabilone [vandf]
25. Nabilone [mart.]
26. Nabilone [who-dd]
27. Schembl33339
28. Nabilone [orange Book]
29. Dea No. 7379
30. Dtxsid8023341
31. Dtxsid401015800
32. Zinc1542930
33. Bdbm50287941
34. Nabilone, Solid, >=98% (hplc)
35. Db00486
36. (+-)-3-(1,1-dimethylheptyl)-6,6abeta,7,8,10,10aalpha-hexahydro-1-hydroxy-6,6-dimethyl-9h-dibenzo(b,d)pyran-9-one
37. (+-)-trans-3-(1,1-dimethylheptyl)-6a,7,8,9,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-6h-dibenzo(b,d)pyran-9-on
38. (+-)-trans-3-(1,1-dimethylheptyl-7,8,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6h-dibenzo(b,d)pyran-9(6ah)-on
39. D05099
40. N-0100
41. 022n710
42. Q419079
43. (+-)-trans-3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-9h-dibenzo(b,d)pyran-9-one
44. (+/-)-3-(1,1-dimethylheptyl-6,6a.beta.,7,8,10,10a.alpha.-hexahydro-1-hydroxy-6,6-dimethyl-9h-dibenzo(b,d)pyran-9-one
45. (-)-trans-1-hydroxy-3-(1,1-dimethylheptyl)-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-9h-dibenzo[b,d]-pyran-9-one
46. (-)-trans-1-hydroxy-3-(1,1-dimethylheptyl)-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-9h-dibenzo[b,d]pyran-9-one
47. (-)-trans-3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-9h-dibenzo[b,d]pyran-9-one
48. (6ar,10ar)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)-6h,6ah,7h,8h,9h,10h,10ah-benzo[c]isochromen-9-one
49. (6ar,10ar)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)-7,8,10,10a-tetrahydro-6h-benzo[c]chromen-9(6ah)-one
50. (6ar,10ar)-3-(1,1-dimethyl-heptyl)-1-hydroxy-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-benzo[c]chromen-9-one
51. (6ar,10ar)-3-(1,1-dimethylheptyl)-1-hydroxy-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-9h-benzo[c]chromen-9-one
52. 61617-09-2
53. 9h-dibenzo(b,d)pyran-9-one, 3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-, Trans-, (+-)-
54. 9h-dibenzo(b,d)pyran-9-one, 3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-, Trans-, (+/-)-
55. Trans-1-hydroxy-3-(1,1-dimethylheptyl)-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-9h-dibenzo[b,d]-pyran-9-one
56. Trans-1-hydroxy-3-(1,1-dimethylheptyl)-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-9h-dibenzo[b,d]pyran-9-one
57. Trans-3-(1',1'-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-9h-dibenzo[b,d]pyran-9-one
| Molecular Weight | 372.5 g/mol |
|---|---|
| Molecular Formula | C24H36O3 |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 372.26644501 g/mol |
| Monoisotopic Mass | 372.26644501 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 524 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cesamet |
| PubMed Health | Nabilone (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | Cesamet (nabilone) is a synthetic cannabinoid for oral administration. Nabilone as a raw material occurs as a white to off-white polymorphic crystalline powder. In aqueous media, the solubility of nabilone is less than 0.5 mg/L, with pH values rang... |
| Active Ingredient | Nabilone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Cesamet |
| PubMed Health | Nabilone (By mouth) |
| Drug Classes | Antiemetic |
| Drug Label | Cesamet (nabilone) is a synthetic cannabinoid for oral administration. Nabilone as a raw material occurs as a white to off-white polymorphic crystalline powder. In aqueous media, the solubility of nabilone is less than 0.5 mg/L, with pH values rang... |
| Active Ingredient | Nabilone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Meda Pharms |
Nabilone is indicated for the treatment of the nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. This restriction is required because a substantial proportion of any group of patients treated with Nabilone can be expected to experience disturbing psychotomimetic reactions not observed with other antiemetic agents.
FDA Label
Nabilone is a cannabinoid with therapeutic uses. It is an analog of dronabinol (also known as tetrahydrocannabinol or THC), the psychoactive ingredient in cannabis. Although structurally distinct from THC, nabilone mimics THC's structure and pharmacological activity through weak partial agonist activity at Cannabinoid-1 (CB1R) and Cannabinoid-2 (CB2R) receptors, however it is considered to be twice as active as -THC.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
A - Alimentary tract and metabolism
A04 - Antiemetics and antinauseants
A04A - Antiemetics and antinauseants
A04AD - Other antiemetics
A04AD11 - Nabilone
Absorption
Nabilone appears to be completely absorbed from the human gastrointestinal tract when administered orally. Following oral administration of a 2 mg dose of radiolabeled nabilone, peak plasma concentrations of approximately 2 ng/mL nabilone and 10 ng equivalents/mL total radioactivity are achieved within 2.0 hours.
Route of Elimination
The route and rate of the elimination of nabilone and its metabolites are similar to those observed with other cannabinoids, including delta-9-THC (dronabinol). When nabilone is administered intravenously, the drug and its metabolites are eliminated mainly in the feces (approximately 67%) and to a lesser extent in the urine (approximately 22%) within 7 days. Of the 67% recovered from the feces, 5% corresponded to the parent compound and 16% to its carbinol metabolite. Following oral administration about 60% of nabilone and its metabolites were recovered in the feces and about 24% in urine. Therefore, it appears that the major excretory pathway is the biliary system.
Volume of Distribution
The apparent volume of distribution of nabilone is about 12.5 L/kg.
Hepatic. Two metabolic pathways have been suggested. The major pathway probably involves the direct oxidation of Nabilone to produce hydroxylic and carboxylic analogues. These compounds are thought to account for the remaining plasma radioactivity when carbinol metabolites have been extracted.
The plasma half-life (T1/2) values for nabilone and total radioactivity of identified and unidentified metabolites are about 2 and 35 hours, respectively.
Nabilone is an orally active synthetic cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS). It has been suggested that the antiemetic effect of nabilone is caused by interaction with the cannabinoid receptor system, i.e., the CB (1) receptor, which is a component of the endocannabinoid system of the body. The endocannabinoid system is widely distributed throughout the central and peripheral nervous system (via the Cannabinoid Receptors CB1 and CB2) and plays a role in many physiological processes such as inflammation, cardiovascular function, learning, pain, memory, stress and emotional regulation, and the sleep/wake cycle among many others. CB1 receptors are found in both the central and peripheral nervous system, and are most abundant in the hippocampus and amygdala, which are the areas of the brain responsible for short-term memory storage and emotional regulation. CB2 receptors are mainly located in the peripheral nervous system and can be found on lymphoid tissue where they are involved in regulation of immune function.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
ABOUT THIS PAGE
39
PharmaCompass offers a list of Nabilone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nabilone manufacturer or Nabilone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nabilone manufacturer or Nabilone supplier.
PharmaCompass also assists you with knowing the Nabilone API Price utilized in the formulation of products. Nabilone API Price is not always fixed or binding as the Nabilone Price is obtained through a variety of data sources. The Nabilone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nabilone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nabilone, including repackagers and relabelers. The FDA regulates Nabilone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nabilone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nabilone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nabilone supplier is an individual or a company that provides Nabilone active pharmaceutical ingredient (API) or Nabilone finished formulations upon request. The Nabilone suppliers may include Nabilone API manufacturers, exporters, distributors and traders.
click here to find a list of Nabilone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nabilone DMF (Drug Master File) is a document detailing the whole manufacturing process of Nabilone active pharmaceutical ingredient (API) in detail. Different forms of Nabilone DMFs exist exist since differing nations have different regulations, such as Nabilone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nabilone DMF submitted to regulatory agencies in the US is known as a USDMF. Nabilone USDMF includes data on Nabilone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nabilone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nabilone suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nabilone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nabilone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nabilone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nabilone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nabilone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nabilone suppliers with NDC on PharmaCompass.
Nabilone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nabilone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nabilone GMP manufacturer or Nabilone GMP API supplier for your needs.
A Nabilone CoA (Certificate of Analysis) is a formal document that attests to Nabilone's compliance with Nabilone specifications and serves as a tool for batch-level quality control.
Nabilone CoA mostly includes findings from lab analyses of a specific batch. For each Nabilone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nabilone may be tested according to a variety of international standards, such as European Pharmacopoeia (Nabilone EP), Nabilone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nabilone USP).
