
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
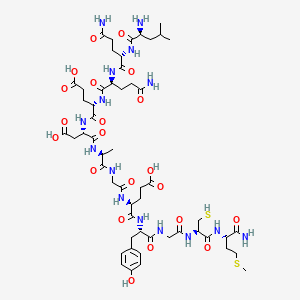

1. Lissamine-rhodamine Dodecanoic Acid
2. Lr-12
3. Motrem
1. Dtxsid201336482
2. 2014384-91-7
| Molecular Weight | 1342.5 g/mol |
|---|---|
| Molecular Formula | C54H83N15O21S2 |
| XLogP3 | -8.3 |
| Hydrogen Bond Donor Count | 20 |
| Hydrogen Bond Acceptor Count | 24 |
| Rotatable Bond Count | 45 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 634 |
| Heavy Atom Count | 92 |
| Formal Charge | 0 |
| Complexity | 2630 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Nangibotide targets the immunoreceptor TREM-1 (triggering receptor expressed on myeloid cells-1), and has been explored in treating inflammatory disorders like septic schock. Safety and pharmacokinetics studies have shown Nangibotide to be safe and well-tolerated in humans, while animal septic shock models have shown its ability to restore vascular function and improve survival. As of July 2020, Inotrem is recuriting patients to study Nangibotide's effects on patients with COVID-19 and systemic inflammation (NCT04429334).
ABOUT THIS PAGE
