
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
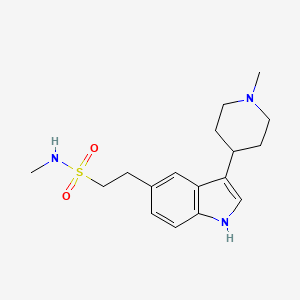

1. 1h-indole-5-ethanesulfonamide, N-methyl-3-(1-methyl-4-piperidinyl)-, Monohydrochloride
2. Amerge
3. Colatan
4. Gr 85548a
5. N-methyl-3-(1-methyl-4-piperidinyl)-1h-indole-5-ethanesulfonamide
6. N-methyl-3-(1-methyl-4-piperidyl)indole-5-ethanesulfonamide Monohydrochloride
7. Naramig
8. Naratriptan Hydrochloride
1. 121679-13-8
2. N-methyl-2-[3-(1-methylpiperidin-4-yl)-1h-indol-5-yl]ethanesulfonamide
3. 1h-indole-5-ethanesulfonamide, N-methyl-3-(1-methyl-4-piperidinyl)-
4. N-methyl-2-(3-(1-methylpiperiden-4-yl)indole-5-yl)ethanesulfonamide
5. Naratriptan (inn)
6. Qx3kxl1za2
7. Chembl1278
8. N-methyl-2-[3-(1-methyl-4-piperidyl)-1h-indol-5-yl]-ethanesulfonamide
9. Chebi:7478
10. Colatan
11. Naratriptan [inn]
12. Naratriptanum
13. Naramig (tn)
14. Ncgc00181786-01
15. Naratriptan [inn:ban]
16. Unii-qx3kxl1za2
17. Naratriptan [mi]
18. Gtpl45
19. Naratriptan [vandf]
20. Schembl68753
21. Naratriptan [who-dd]
22. Bidd:gt0312
23. Zinc4076
24. Dtxsid7023354
25. Naratriptan [orange Book]
26. Hy-b0197
27. Bdbm50073682
28. N-methyl-2-[3-(1-methyl-4-piperidyl)-1h-indol-5-yl]ethanesulfonamide
29. Akos015895854
30. Ac-5013
31. Bcp9000978
32. Db00952
33. Ncgc00181786-03
34. Ncgc00181786-04
35. Ncgc00181786-08
36. Ft-0656760
37. A25336
38. C07792
39. D08255
40. Ab01565792_02
41. L000432
42. Q421315
43. N-methyl-3-(1-methyl-4 -piperidinyl)-1h-indole-5-ethansulphonamide
44. N-methyl-3-(1-methyl-4-piperidinyl)-1h-indole-5-ethanesulphonamide
45. 2-[3-(1-methyl-piperidin-4-yl)-1h-indol-5-yl]-ethanesulfonic Acid Methylamide
46. N-methyl-2-[3-(1-methylpiperidin-4-yl)-1h-indol-5-yl]ethane-1-sulfonamide
| Molecular Weight | 335.5 g/mol |
|---|---|
| Molecular Formula | C17H25N3O2S |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 335.16674822 g/mol |
| Monoisotopic Mass | 335.16674822 g/mol |
| Topological Polar Surface Area | 73.6 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 483 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Amerge |
| PubMed Health | Naratriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | AMERGE contains naratriptan hydrochloride, a selective 5-HT1B/1D receptor agonist. Naratriptan hydrochloride is chemically designated as N-methyl-3-(1-methyl-4-piperidinyl)-1H-indole-5-ethanesulfonamide monohydrochloride, and it has the following str... |
| Active Ingredient | Naratriptan hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 2.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 4 | |
|---|---|
| Drug Name | Naratriptan |
| PubMed Health | Naratriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | Naratriptan tablets, USP contain naratriptan as the hydrochloride, which is a selective 5-hydroxytryptamine1 receptor subtype agonist. Naratriptan hydrochloride, USP is chemically designated as N-methyl-3-(1-methyl-4-piperidinyl)-1H-indole-5-ethanesu... |
| Active Ingredient | Naratriptan hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 2.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Mylan Pharms; Apotex; Sun Pharm Inds; Sandoz; Roxane; Teva Pharms; Paddock; Orchid Hlthcare; Heritage Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Amerge |
| PubMed Health | Naratriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | AMERGE contains naratriptan hydrochloride, a selective 5-HT1B/1D receptor agonist. Naratriptan hydrochloride is chemically designated as N-methyl-3-(1-methyl-4-piperidinyl)-1H-indole-5-ethanesulfonamide monohydrochloride, and it has the following str... |
| Active Ingredient | Naratriptan hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 2.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 4 of 4 | |
|---|---|
| Drug Name | Naratriptan |
| PubMed Health | Naratriptan (By mouth) |
| Drug Classes | Antimigraine |
| Drug Label | Naratriptan tablets, USP contain naratriptan as the hydrochloride, which is a selective 5-hydroxytryptamine1 receptor subtype agonist. Naratriptan hydrochloride, USP is chemically designated as N-methyl-3-(1-methyl-4-piperidinyl)-1H-indole-5-ethanesu... |
| Active Ingredient | Naratriptan hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 2.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Mylan Pharms; Apotex; Sun Pharm Inds; Sandoz; Roxane; Teva Pharms; Paddock; Orchid Hlthcare; Heritage Pharms |
For the acute treatment of migraine attacks with or without aura in adults.
FDA Label
Naratriptan is a selective agonist of serotonin (5-hydroxytryptamine; 5-HT) type 1B and 1D receptors. It is structurally and pharmacologically related to other selective 5-HT1B/1D receptor agonist. Naratriptan has only a weak affinity for 5-HT1A, 5-HT5A, and 5-HT7 receptors and no significant affinity or pharmacological activity at 5-HT2, 5-HT3 or 5-HT4 receptor subtypes or at alpha1-, alpha2-, or beta-adrenergic, dopamine1,; dopamine2; muscarinic, or benzodiazepine receptors. This action in humans correlates with the relief of migraine headache. In addition to causing vasoconstriction, experimental data from animal studies show that Naratriptan also activates 5-HT1 receptors on peripheral terminals of the trigeminal nerve innervating cranial blood vessels, which may also contribute to the antimigrainous effect of Naratriptan in humans.
Serotonin 5-HT1 Receptor Agonists
Endogenous compounds and drugs that specifically stimulate SEROTONIN 5-HT1 RECEPTORS. Included under this heading are agonists for one or more of the specific 5-HT1 receptor subtypes. (See all compounds classified as Serotonin 5-HT1 Receptor Agonists.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
N02CC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N02 - Analgesics
N02C - Antimigraine preparations
N02CC - Selective serotonin (5ht1) agonists
N02CC02 - Naratriptan
Absorption
Well absorbed (74% oral biovaility), absorption is rapid with peak plasma concentrations after 2-5 hours. The rate of absorption is slower during a migraine attack.
Volume of Distribution
170 L
Clearance
6.6 mL/min/kg
Primarily hepatic. In vitro, naratriptan is metabolized by a wide range of cytochrome P450 isoenzymes into a number of inactive metabolites.
5-8 hours
Three distinct pharmacological actions have been implicated in the antimigraine effect of the triptans: (1) stimulation of presynaptic 5-HT1D receptors, which serves to inhibit both dural vasodilation and inflammation; (2) direct inhibition of trigeminal nuclei cell excitability via 5-HT1B/1D receptor agonism in the brainstem and (3) vasoconstriction of meningeal, dural, cerebral or pial vessels as a result of vascular 5-HT1B receptor agonism.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
53
PharmaCompass offers a list of Naratriptan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Naratriptan manufacturer or Naratriptan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Naratriptan manufacturer or Naratriptan supplier.
PharmaCompass also assists you with knowing the Naratriptan API Price utilized in the formulation of products. Naratriptan API Price is not always fixed or binding as the Naratriptan Price is obtained through a variety of data sources. The Naratriptan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Naratriptan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Naratriptan, including repackagers and relabelers. The FDA regulates Naratriptan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Naratriptan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Naratriptan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Naratriptan supplier is an individual or a company that provides Naratriptan active pharmaceutical ingredient (API) or Naratriptan finished formulations upon request. The Naratriptan suppliers may include Naratriptan API manufacturers, exporters, distributors and traders.
click here to find a list of Naratriptan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Naratriptan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Naratriptan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Naratriptan GMP manufacturer or Naratriptan GMP API supplier for your needs.
A Naratriptan CoA (Certificate of Analysis) is a formal document that attests to Naratriptan's compliance with Naratriptan specifications and serves as a tool for batch-level quality control.
Naratriptan CoA mostly includes findings from lab analyses of a specific batch. For each Naratriptan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Naratriptan may be tested according to a variety of international standards, such as European Pharmacopoeia (Naratriptan EP), Naratriptan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Naratriptan USP).
