
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
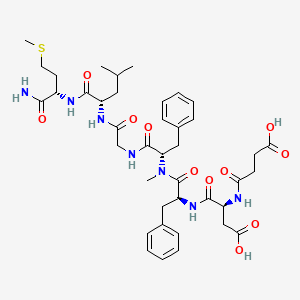

1. Substance P (6-11), Succinyl-asp(6)-me-phe(8)-
2. Substance P (6-11), Succinyl-aspartyl(6)-methylphenylalanine(8)-
3. Succinyl-6-asp-8-me-phe-substance P (6-11)
1. 106128-89-6
2. Chembl106124
3. (5s,8s,14s,17s,20s)-14,17-dibenzyl-5-carbamoyl-20-(carboxymethyl)-8-isobutyl-15-methyl-7,10,13,16,19,22-hexaoxo-2-thia-6,9,12,15,18,21-hexaazapentacosan-25-oic Acid
4. Succinyl-6-asp-8-me-phe-substance P (6-11)
5. Substance P (6-11), Succinyl-asp(6)-me-phe(8)-
6. Substance P (6-11), Succinyl-aspartyl(6)-methylphenylalanine(8)-
7. Gtpl2127
8. Schembl5238634
9. Dtxsid80909926
10. Suc[asp6,mephe3]sp(6-11)
11. Hy-p0187
12. Suc[asp6,mephe3]sp(6-11))
13. Bdbm50052524
14. Mfcd00076799
15. Zinc95615286
16. Cs-8091
17. (3s)-4-[[(2s)-1-[[(2s)-1-[[2-[[(2s)-1-[[(2s)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-1-oxo-3-phenylpropan-2-yl]-methylamino]-1-oxo-3-phenylpropan-2-yl]amino]-3-[(4-hydroxy-4-oxobutanoyl)amino]-4-oxobutanoic Acid
18. 4-10-neuromedin B (swine Spinal Cord), N-(3-carboxy-1-oxopropyl)-6-(n-methyl-l-phenylalanine)-7-de-l-valine-
19. L-methioninamide, N-(3-carboxy-1-oxopropyl)-l-alpha-aspartyl-l-phenylalanyl-n-methyl-l-phenylalanylglycyl-l-leucyl-
20. N-(3-carboxy-1-oxopropyl)-6-(n-methyl-l-phenylalanine)-7-de-l-valine-4-10-neuromedin B (swine Spinal Cord)
21. B6580
22. A16945
23. Succinyl-(asp6,n-me-phe8)-substance P (6-11)
24. J-001543
25. Q27088780
26. (3s)-4-[[(2s)-1-[[(2s)-1-[[2-[[(2s)-1-[[(2s)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-1-oxo-3-phenylpropan-2-yl]-methylamino]-1-oxo-3-phenylpropan-2-yl]amino]-3-(3-carboxypropanoylamino)-4-oxobutanoic Acid
27. (s)-n-((s)-1-{[(s)-1-({[(s)-1-((s)-1-carbamoyl-3-methylsulfanyl-propylcarbamoyl)-3-methyl-butylcarbamoyl]-methyl}-carbamoyl)-2-phenyl-ethyl]-methyl-carbamoyl}-2-phenyl-ethyl)-3-(3-carboxy-propionylamino)-succinamic Acid
28. 4-({14-benzyl-7,10,13-trihydroxy-5-[hydroxy(imino)methyl]-15-methyl-8-(2-methylpropyl)-16-oxo-18-phenyl-2-thia-6,9,12,15-tetraazaoctadeca-6,9,12-trien-17-yl}imino)-3-[(3-carboxy-1-hydroxypropylidene)amino]-4-hydroxybutanoic Acid
| Molecular Weight | 842.0 g/mol |
|---|---|
| Molecular Formula | C40H55N7O11S |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 26 |
| Exact Mass | 841.36802677 g/mol |
| Monoisotopic Mass | 841.36802677 g/mol |
| Topological Polar Surface Area | 309 Ų |
| Heavy Atom Count | 59 |
| Formal Charge | 0 |
| Complexity | 1450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : USA
Brand Name : TYSABRI
Dosage Form : VIAL; SINGLE-USE
Dosage Strength : 300MG
Packaging :
Approval Date :
Application Number : 125104
Regulatory Info :
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Tysabri
Dosage Form : CONCENTRATE FOR SOLUTION FOR INFUSION
Dosage Strength : 300 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Tysabri
Dosage Form : Inf Conc
Dosage Strength : 300mg/15ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Tysabri
Dosage Form : Inj L?s
Dosage Strength : 150mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : TYSABRI
Dosage Form : SOLUTION
Dosage Strength : 300MG/15ML
Packaging : 1VIAL
Approval Date :
Application Number : 2286386
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Tysabri
Dosage Form :
Dosage Strength :
Packaging : 2
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Tysabri
Dosage Form :
Dosage Strength :
Packaging : 2
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Tysabri
Dosage Form :
Dosage Strength :
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Tysabri
Dosage Form :
Dosage Strength :
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : USA
Brand Name : TYRUKO
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 300MG/15ML(20MG/ML)
Packaging :
Approval Date :
Application Number : 761322
Regulatory Info :
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?