

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
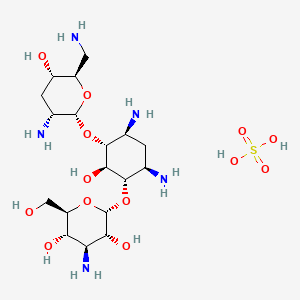

1. Brulamycin
2. Nebcin
3. Nebicin
4. Nebramycin Factor 6
5. Obracin
6. Sulfate, Tobramycin
7. Tobracin
8. Tobramycin
1. 49842-07-1
2. Tobramycin Sulphate
3. Nebcin
4. Tobramycinsulfate
5. 79645-27-5
6. Mls000069826
7. Smr000058891
8. Dartobcin
9. Nebcine
10. Tobraneg
11. Tobrasix
12. (2s,3r,4s,5s,6r)-4-amino-2-{[(1s,2s,3r,4s,6r)-4,6-diamino-3-{[(2r,3r,5s,6r)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy}-2-hydroxycyclohexyl]oxy}-6-(hydroxymethyl)oxane-3,5-diol Sulfate
13. Opera_id_1050
14. Schembl2837
15. Chembl1200780
16. Hms2230j04
17. Mfcd00133864
18. Akos025310614
19. 842t071
20. A923784
21. Tobramycin Sulfate Salt, Aminoglycoside Antibiotic
22. Q-201838
23. 2-ethoxy-5-[(morpholine-4-carbonyl)-amino]-benzenesulfonylchloride
24. (2s,3r,4s,5s,6r)-4-amino-2-(((1s,2s,3r,4s,6r)-4,6-diamino-3-(((2r,3r,5s,6r)-3-amino-6-(aminomethyl)-5-hydroxytetrahydro-2h-pyran-2-yl)oxy)-2-hydroxycyclohexyl)oxy)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,5-diol Sulfate
25. (2s,3r,4s,5s,6r)-4-amino-2-((1s,2s,3r,4s,6r)-4,6-diamino-3-((2r,3r,5s,6r)-3-amino-6-(aminomethyl)-5-hydroxytetrahydro-2h-pyran-2-yloxy)-2-hydroxycyclohexyloxy)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,5-diol Sulfate
26. (2s,3r,4s,5s,6r)-4-amino-2-[(1s,2s,3r,4s,6r)-4,6-diamino-3-[(2r,3r,5s,6r)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol,sulfuric Acid
| Molecular Weight | 565.6 g/mol |
|---|---|
| Molecular Formula | C18H39N5O13S |
| Hydrogen Bond Donor Count | 12 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 6 |
| Exact Mass | 565.22650749 g/mol |
| Monoisotopic Mass | 565.22650749 g/mol |
| Topological Polar Surface Area | 351 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 691 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 14 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 3 | |
|---|---|
| Drug Name | TOBRAMYCIN SULFATE |
| Active Ingredient | TOBRAMYCIN SULFATE |
| Company | AKORN (Application Number: A205179); BAXTER HLTHCARE CORP (Application Number: A206965); FRESENIUS KABI USA (Application Number: A065122); FRESENIUS KABI USA (Application Number: N050789); HOSPIRA (Application Number: A063111); HOSPIRA (Application Number: A063112); MYLAN LABS LTD (Application Number: A065407); TEVA PHARMS USA (Application Number: A063100); WEST-WARD PHARMS INT (Application Number: A063117); X GEN PHARMS (Application Number: A065013); XELLIA PHARMS APS (Application Number: A205685) |
| 2 of 3 | |
|---|---|
| Drug Name | TOBRAMYCIN SULFATE (PHARMACY BULK) |
| Active Ingredient | TOBRAMYCIN SULFATE |
| Company | FRESENIUS KABI USA (Application Number: A065120) |
| 3 of 3 | |
|---|---|
| Drug Name | TOBRAMYCIN SULFATE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER |
| Active Ingredient | TOBRAMYCIN SULFATE |
| Company | HOSPIRA (Application Number: A063081) |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
