
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Ag 1343
2. Ag-1343
3. Ag1343
4. Mesylate, Nelfinavir
5. Monomethane Sulfonate, Nelfinavir
6. Nelfinavir Mesylate
7. Nelfinavir Monomethane Sulfonate
8. Sulfonate, Nelfinavir Monomethane
9. Viracept
1. 159989-64-7
2. Viracept
3. Ag-1343
4. Viracept (tn)
5. Nelfinavir (inn)
6. Ho3ogh5d7i
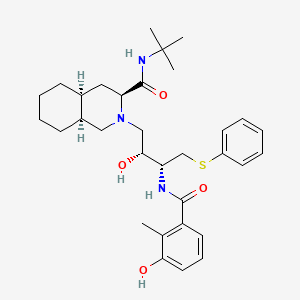
7. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1h-isoquinoline-3-carboxamide
8. Nsc-747167
9. Chebi:7496
10. 1un
11. 2-[2-hydroxy-3-(3-hydroxy-2-methyl-benzoylamino)-4-phenyl Sulfanyl-butyl]-decahydro-isoquinoline-3-carboxylic Acid Tert-butylamide
12. Nlf
13. Nelfinavir [inn]
14. Nelfinavir [inn:ban]
15. Nfv
16. (3s,4as,8as)-n-(tert-butyl)-2-((2r,3r)-2-hydroxy-3-(3-hydroxy-2-methylbenzamido)-4-(phenylthio)butyl)decahydroisoquinoline-3-carboxamide
17. Chembl1205
18. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methyl-benzoyl)amino]-4-phenylsulfanyl-butyl]-3,4,4a,5,6,7,8,8a-octahydro-1h-isoquinoline-3-carboxamide
19. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylphenyl)formamido]-4-(phenylsulfanyl)butyl]-decahydroisoquinoline-3-carboxamide
20. 3-isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2-((2r,3r)-2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-, (3s,4as,8as)-
21. Smr000596515
22. Nsc722664
23. Ncgc00090782-03
24. Nelfinavir Mesylate Ag1343
25. Unii-ho3ogh5d7i
26. Met-sdf-1.beta. & Nelfinavir
27. 1ohr
28. 3-isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-, (3s,4as,8as)-
29. (3s-(2(2s*,3s*),3alpha,4abeta,8abeta))-n-(1,1-dimethylethyl)decahydro-2-(2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-3-isoquinolinecarboxamide
30. Nelfinavir [mi]
31. Nelfinavir [vandf]
32. Chembl584
33. Nelfinavir [who-dd]
34. Bidd:pxr0143
35. Schembl38218
36. Mls001195634
37. Mls001304729
38. N-(1,1-dimethylethyl)decahydro-2-(2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-3-isoquinolinecarboxamide (3s-(2(2s*,3s*),3alpha,4abeta,8abeta))-
39. Bidd:gt0395
40. Nelfinavir [ema Epar]
41. Dtxsid5035080
42. Gtpl11090
43. Hms2232i04
44. Met-stromal Cell-derived Factor-1.beta. (human) & Nelfinavir
45. Zinc3833846
46. Bdbm50061306
47. Mfcd01938163
48. Nsc747167
49. Akos000280862
50. Am84529
51. Cs-0677
52. Db00220
53. Nsc 747167
54. Mrf-0000208
55. Ncgc00090782-04
56. Ncgc00090782-05
57. Ncgc00090782-17
58. 3-isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2-(2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-, (3s-(2-(2s*,3s*),3-alpha,4a-beta,8a-beta))-
59. Ac-20032
60. As-75258
61. Hy-15287
62. 89n647
63. C07257
64. C73027
65. D08259
66. Ab00698259-15
67. A810095
68. A810096
69. Q423366
70. (3s, 4as, 8as)-2-[(2r, 3r)-2-hydroxy-3-(3-hydroxy-2-methyl-benzoylamino)-4-phenylthiobutyl]decahydroisoquinoline-3-carboxylic Acid T-butylamide
71. (3s, 4as, 8as)-2-[(2r, 3r)-2-hydroxy-3-(3-hydroxy-2-methylbenzoylamino)-4-phenylthiobutyl]decahydroisoquinoline-3-carboxylic Acid T-butylamide
72. (3s,4abeta,8abeta)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-(2-methyl-3-hydroxybenzoylamino)-4-(phenylthio)butyl]decahydroisoquinoline-3alpha-carboxamide
73. (3s,4as,8as)-2-[(2r,3r)-2-hydroxy-3-(3-hydroxy-2-methyl-benzoylamino)-4-phenylsulfanyl-butyl]-decahydro-isoquinoline-3-carboxylic Acid Tert-butylamide; Compound With Methanesulfonic Acid
74. (3s,4as,8as)-2-[(2r,3r)-2-hydroxy-3-(3-hydroxy-2-methylbenzoylamino)-4-phenylthiobutyl]decahydroisoquinoline-3-carboxylic Acid T-butylamide
75. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylsulfanyl)butyl]decahydroisoquinoline-3-carboxamide
76. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-[[(3-hydroxy-2-methylphenyl)-oxomethyl]amino]-4-(phenylthio)butyl]-3,4,4a,5,6,7,8,8a-octahydro-1h-isoquinoline-3-carboxamide
77. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-2-hydroxy-3-{[(3-hydroxy-2-methylphenyl)carbonyl]amino}-4-(phenylsulfanyl)butyl]decahydroisoquinoline-3-carboxamide
78. (3s,4as,8as)-n-tert-butyl-2-[(2r,3r)-3-[(2-methyl-3-oxidanyl-phenyl)carbonylamino]-2-oxidanyl-4-phenylsulfanyl-butyl]-3,4,4a,5,6,7,8,8a-octahydro-1h-isoquinoline-3-carboxamide
79. 3-isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2- [(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-, (3s,4as,8as)-
80. 3-isoquinolinecarboxamide,1-dimethyl Ethyl)decahydro-2-[(2r,3r)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-, (3s,4as,8as)-
81. 3-isoquinolinecarboxamide,1-dimethylethyl)decahydro-2-[2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-, [3s-[2(2s*,3s*),3.alpha.,4a.beta.,8a.beta.]]-
82. N-(tert-butyl)-2-(2-hydroxy-3-(3-hydroxy-2-methylbenzamido)-4-(phenylthio)butyl)decahydroisoquinoline-3-carboxamide
| Molecular Weight | 567.8 g/mol |
|---|---|
| Molecular Formula | C32H45N3O4S |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 10 |
| Exact Mass | 567.31307810 g/mol |
| Monoisotopic Mass | 567.31307810 g/mol |
| Topological Polar Surface Area | 127 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 830 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Viracept |
| PubMed Health | Nelfinavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRACEPT (nelfinavir mesylate) is an inhibitor of the human immunodeficiency virus (HIV) protease.VIRACEPT Tablets are available for oral administration as a light blue, capsule-shaped tablet with a clear film coating in 250 mg strength (as nelfi... |
| Active Ingredient | Nelfinavir mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 625mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Agouron |
| 2 of 2 | |
|---|---|
| Drug Name | Viracept |
| PubMed Health | Nelfinavir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRACEPT (nelfinavir mesylate) is an inhibitor of the human immunodeficiency virus (HIV) protease.VIRACEPT Tablets are available for oral administration as a light blue, capsule-shaped tablet with a clear film coating in 250 mg strength (as nelfi... |
| Active Ingredient | Nelfinavir mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 625mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Agouron |
Used in combination with other antiviral drugs in the treatment of HIV in both adults and children.
FDA Label
Viracept is indicated in antiretroviral combination treatment of human-immunodeficiency-virus (HIV-1)-infected adults, adolescents and children of three years of age and older.
In protease-inhibitor (PI)-experienced patients, the choice of nelfinavir should be based on individual viral resistance testing and treatment history.
Nelfinavir is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Nelfinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
J05AE04
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AE - Protease inhibitors
J05AE04 - Nelfinavir
Absorption
Well absorbed following oral administration.
Route of Elimination
The terminal half-life in plasma was typically 3.5 to 5 hours. The majority (87%) of an oral 750 mg dose containing 14C-nelfinavir was recovered in the feces; fecal radioactivity consisted of numerous oxidative metabolites (78%) and unchanged nelfinavir (22%). Only 12% of the dose was recovered in urine, of which unchanged nelfinavir was the major component.
Volume of Distribution
2 to 7 L/kg
Primarily hepatic via cytochrome P450 (CYP450) enzymes. CYP3A and CYP2C19 appear to be the predominant enzymes that metabolize nelfinavir in humans. One major and several minor metabolites are found in plasma; the major oxidative metabolite has in vitro antiviral activity comparable to that of the parent drug.
Nelfinavir has known human metabolites that include 3,4-Dihydroxynelfinavir and Nelfinavir hydroxyl-t- butylamide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
3.5 - 5 hours
Nelfinavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles.
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Vira-Cept
Dosage Form : TAB
Dosage Strength : 250mg
Packaging : 270X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Vira-Cept 50Mg/G Powder
Dosage Form : POW
Dosage Strength : 50mg/g
Packaging : 270X1mg
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : NEL – 250
Dosage Form : Tablet
Dosage Strength : 250MG
Packaging : 1x60 HDPE bottles
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 1x60 HDPE bottles
Regulatory Info :
Dosage : Tablet
Dosage Strength : 250MG
Brand Name : NEL – 250
Approval Date :
Application Number :
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

ANALYTICAL

ABOUT THIS PAGE
14
PharmaCompass offers a list of Nelfinavir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nelfinavir manufacturer or Nelfinavir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nelfinavir manufacturer or Nelfinavir supplier.
PharmaCompass also assists you with knowing the Nelfinavir API Price utilized in the formulation of products. Nelfinavir API Price is not always fixed or binding as the Nelfinavir Price is obtained through a variety of data sources. The Nelfinavir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nelfinavir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nelfinavir, including repackagers and relabelers. The FDA regulates Nelfinavir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nelfinavir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Nelfinavir supplier is an individual or a company that provides Nelfinavir active pharmaceutical ingredient (API) or Nelfinavir finished formulations upon request. The Nelfinavir suppliers may include Nelfinavir API manufacturers, exporters, distributors and traders.
click here to find a list of Nelfinavir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nelfinavir DMF (Drug Master File) is a document detailing the whole manufacturing process of Nelfinavir active pharmaceutical ingredient (API) in detail. Different forms of Nelfinavir DMFs exist exist since differing nations have different regulations, such as Nelfinavir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nelfinavir DMF submitted to regulatory agencies in the US is known as a USDMF. Nelfinavir USDMF includes data on Nelfinavir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nelfinavir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nelfinavir suppliers with USDMF on PharmaCompass.
Nelfinavir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nelfinavir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nelfinavir GMP manufacturer or Nelfinavir GMP API supplier for your needs.
A Nelfinavir CoA (Certificate of Analysis) is a formal document that attests to Nelfinavir's compliance with Nelfinavir specifications and serves as a tool for batch-level quality control.
Nelfinavir CoA mostly includes findings from lab analyses of a specific batch. For each Nelfinavir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nelfinavir may be tested according to a variety of international standards, such as European Pharmacopoeia (Nelfinavir EP), Nelfinavir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nelfinavir USP).
