

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
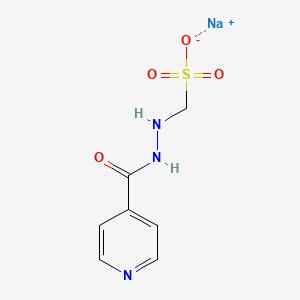

1. Isoniazid Methanesulfonate
2. Methaniazide
3. Methaniazide, Sodium Salt
4. Neoiscotin
1. Neoiscotin
2. Methaniazide Sodium
3. 3804-89-5
4. Isoniazid Methanesulfonate Sodium
5. Neo-tizide Sodium Salt
6. Neotizide Sodium Salt
7. Sodium Isoniazid Methanesulfonate
8. Sodium Methanesulfonate Isoniazid
9. 7sz6s9m7ig
10. Isonicotinic Acid, 2-(sulfomethyl)hydrazide Monosodium Salt
11. Sodium;[2-(pyridine-4-carbonyl)hydrazinyl]methanesulfonate
12. Nsc-81103
13. Ncgc00181167-01
14. Einecs 223-275-0
15. Nsc 81103
16. Unii-7sz6s9m7ig
17. 2'-(sulphomethyl)isonicotinohydrazide, Monosodium Salt
18. Sodium Isonicotinyl Hydrazine Methansulfonate
19. Isonicotinic Acid Hydrazide Methane Sulfonate (van)
20. Isonicotinsaeur-hydrazid-methansulfonsaeuren Natrium [german]
21. Isonicotinic Acid, 2-(sulfomethyl)hydrazide, Sodium Salt
22. 4-pyridinecarboxylic Acid, 2-(sulfomethyl)hydrazide, Monosodium Salt
23. Dsstox_cid_26885
24. Dsstox_rid_81988
25. Dsstox_gsid_46885
26. Isonicotinsaeur-hydrazid-methansulfonsaeuren Natrium
27. Chembl3187762
28. Dtxsid7046885
29. Tox21_112768
30. Methaniazide Sodium [who-dd]
31. Akos033878769
32. Cas-3804-89-5
33. Isoniazid Methanesulfonate Sodium [mi]
34. Sodium Isonicotinylhydrazide Methanesulfonate
35. Q27268806
36. Sodium [(pyridin-4-yl)formohydrazido]methanesulfonate
37. Z2373906036
| Molecular Weight | 253.21 g/mol |
|---|---|
| Molecular Formula | C7H8N3NaO4S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 253.01332120 g/mol |
| Monoisotopic Mass | 253.01332120 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 310 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
