
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
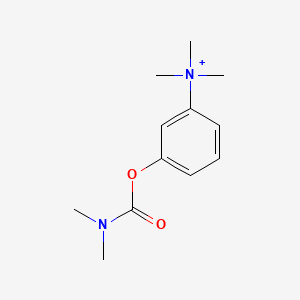

1. Bromide, Neostigmine
2. Methylsulfate, Neostigmine
3. Neostigmine Bromide
4. Neostigmine Methylsulfate
5. Polstigmine
6. Proserine
7. Prostigmin
8. Prostigmine
9. Prozerin
10. Synstigmin
11. Syntostigmine
1. Eustigmin
2. Eustigmine
3. Prostigmine
4. Vagostigmine
5. 59-99-4
6. Prostigmin
7. Juvastigmin
8. M-trimethylammoniumphenyldimethylcarbamate
9. Neostigmine [ban]
10. 3-trimethylammoniumphenyl N,n-dimethylcarbamate
11. 3-[(dimethylcarbamoyl)oxy]-n,n,n-trimethylanilinium
12. Neostigmine Ion
13. Neostigmine Cation
14. Neostigmine (ban)
15. Neostigmine (cation)
16. (m-hydroxyphenyl)trimethylammonium Dimethylcarbamate
17. Prostigmin (tn)
18. Neostigmin
19. [3-(dimethylcarbamoyloxy)phenyl]-trimethylazanium
20. Benzenaminium, 3-(((dimethylamino)carbonyl)oxy)-n,n,n-trimethyl-
21. Chebi:7514
22. Chembl278020
23. Neostigminum
24. 3982twq96g
25. [3-(dimethylcarbamoyloxy)phenyl]-trimethyl-azanium Bromide
26. 3-{[(dimethylamino)carbonyl]oxy}-n,n,n-trimethylanilinium
27. 3-((dimethylcarbamoyl)oxy)-n,n,n-trimethylbenzenaminium
28. Benzenaminium, 3-[[(dimethylamino)carbonyl]oxy]-n,n,n-trimethyl-
29. [3-(dimethylcarbamoyloxy)phenyl]-trimethyl-azanium; Sulfonatooxymethane
30. Ccris 3079
31. Hsdb 3921
32. Ncgc00163240-01
33. Cas-114-80-7
34. Brn 3615946
35. Unii-3982twq96g
36. (m-hydroxyphenyl)trimethylammonium Dimethylcarbamate (ester)
37. Ammonium, (m-hydroxyphenyl)trimethyl-, Dimethylcarbamate (ester)
38. Spectrum_001061
39. Neostigmine [mi]
40. Prestwick0_000468
41. Prestwick1_000468
42. Prestwick2_000468
43. Prestwick3_000468
44. Spectrum2_001278
45. Spectrum4_000072
46. Spectrum5_001234
47. Lopac-n-2001
48. Neostigmine [hsdb]
49. Bmse000762
50. Neostigmine [vandf]
51. Neostigmine [mart.]
52. Lopac0_000816
53. Schembl34419
54. Bspbio_000576
55. Kbiogr_000623
56. Kbioss_001541
57. [3-(dimethylcarbamoyloxy)phenyl]-trimethyl-azanium
58. Divk1c_000165
59. Divk1c_000198
60. Spbio_001276
61. Spbio_002515
62. Bpbio1_000634
63. Gtpl8993
64. Zinc1792
65. Dtxsid1023360
66. Alwkgypquaplqc-uhfffaoysa-
67. Kbio1_000165
68. Kbio1_000198
69. Kbio2_001541
70. Kbio2_004109
71. Kbio2_006677
72. Ninds_000165
73. Ninds_000198
74. Hms2089a22
75. Bdbm50022775
76. Stl058953
77. Akos005711366
78. Ccg-204900
79. Db01400
80. Idi1_000165
81. Idi1_000198
82. Mls-0002855
83. Ncgc00015730-01
84. Ncgc00015730-02
85. Ncgc00015730-03
86. Ncgc00015730-04
87. Ncgc00015730-05
88. Ncgc00015730-06
89. Ncgc00015730-07
90. Ncgc00015730-08
91. Ncgc00015730-09
92. Ncgc00015730-24
93. Ncgc00021658-03
94. Sbi-0050793.p004
95. Ab00053807
96. C07258
97. D08261
98. Ab00053807-25
99. Ab00053807-26
100. Ab00053807_27
101. Ab00053807_28
102. Ab00053807_29
103. Q410546
104. Brd-k18922609-004-04-1
105. Brd-k18922609-004-14-0
| Molecular Weight | 223.29 g/mol |
|---|---|
| Molecular Formula | C12H19N2O2+ |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 223.144652853 g/mol |
| Monoisotopic Mass | 223.144652853 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 1 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cholinesterase Inhibitors; Parasympathomimetics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...ANTI-CHE /ANTICHOLINESTERASE/ AGENTS ARE OF GREAT VALUE IN MGMNT OF PRIMARY /GLAUCOMA/ AS WELL AS OF CERTAIN CATEGORIES OF SECONDARY TYPE (EG APHAKIC GLAUCOMA, FOLLOWING CATARACT EXTRACTION); THE CONGENITAL TYPE RARELY RESPONDS TO OTHER THAN SURGICAL TREATMENT. PRIMARY GLAUCOMA IS SUBDIVIDED INTO NARROW-ANGLE (ACUTE CONGESTIVE) AND WIDE-ANGLE (CHRONIC SIMPLE) TYPES... ANTI-CHE AGENTS PRODUCE A FALL IN INTRAOCULAR PRESSURE IN BOTH TYPES...BY LOWERING THE RESISTANCE TO OUTFLOW OF THE AQAEOUS HUMOR. ... /IN/ ACUTE CONGESTIVE GLAUCOMA...AN ANTI-CHE AGENT IS INSTILLED IN THE CONJUNCTIVAL SAC IN COMBINATION WITH PARASYMPATHOMIMETIC AGENT... CHRONIC SIMPLE...AND SECONDARY GLAUCOMA REQUIRE CAREFUL CONSIDERATION OF THE NEEDS OF THE INDIVIDUAL PT IN SELECTING DRUG OR COMBINATION OF DRUGS... CHOICES AVAIL INCL...ANTICHOLINESTERASE AGENTS...
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 113
...IS USED FOR THE RELIEF OF ABDOMINAL DISTENTION FROM A VARIETY OF MEDICAL AND SURGICAL CAUSES. ...IS TO BE VIEWED MAINLY AS ADJUVANT AGENT IN THE TREATMENT OF DISTENTION. ...WHEN NEOSTIGMINE IS EMPLOYED FOR THE TREATMENT OF ATONY OF THE DETRUSOR MUSCLE OF THE URINARY BLADDER, POSTOPERATIVE DYSURIA IS RELIEVED AND THE TIME INTERVAL BETWEEN OPERATION AND SPONTANEOUS URINATION IS SHORTENED.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 113
...NEOSTIGMINE IS USED IN THE DIFFERENTIAL DIAGNOSIS OF MYASTHENIC CRISIS, IN WHICH CASE IT WILL IMPROVE MUSCLE FUNCTION, AND CHOLINERGIC CRISES, IN WHICH CASE IT WILL WORSEN FUNCTION, AND TO DIAGNOSE MYOTONIA CONGENITA.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 839
For more Therapeutic Uses (Complete) data for NEOSTIGMINE (9 total), please visit the HSDB record page.
...NEOSTIGMINE, MUST BE USED CAUTIOUSLY IN PATIENTS WITH CARDIAC DYSRHYTHMIAS OR BRONCHIAL ASTHMA.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 426
THE DRUG SHOULD NOT BE USED WHEN THERE IS MECHANICAL OBSTRUCTION OF THE INTESTINE OR URINARY BLADDER, WHEN PERITONITIS IS PRESENT, OR WHEN THE VIABILITY OF THE BOWEL IS DOUBTFUL.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 113
The type of reaction to neostigmine in patients with neuromuscular disease is unpredictable. Long-lasting muscle weakness was produced in a 57-yr-old female with dystrophia myotonica. A 50-yr-old male with a 30-yr history of progressive muscle dystrophy exhibited a tonic response to neostigmine during recovery from partial neuromuscular block.
Buzello W et al; Hazards of neostigmine in patients with neuromuscular disorders; Br J Anaesth 54 (5): 529-34 (1982)
Neostigmine in clinical doses can produce an acetylcholine-induced block which can be a potential hazard in anesthetic practice. Study reveals effects of neostigmine in 26 patients anesthetized with thiopentone and nitrous oxide.
Payne JP et al; Neuromuscular blockade by neostigmine in anaesthetized man; Br J Anaesth 52 (1): 69-76 (1980)
For more Drug Warnings (Complete) data for NEOSTIGMINE (6 total), please visit the HSDB record page.
Neostigmine is used for the symptomatic treatment of myasthenia gravis by improving muscle tone.
Neostigmine is a cholinesterase inhibitor used in the treatment of myasthenia gravis and to reverse the effects of muscle relaxants such as gallamine and tubocurarine. Neostigmine, unlike physostigmine, does not cross the blood-brain barrier. By inhibiting acetylcholinesterase, more acetylcholine is available in the synapse, therefore, more of it can bind to the fewer receptors present in myasthenia gravis and can better trigger muscular contraction.
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)
N07AA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N07 - Other nervous system drugs
N07A - Parasympathomimetics
N07AA - Anticholinesterases
N07AA01 - Neostigmine
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EB - Parasympathomimetics
S01EB06 - Neostigmine
Absorption
Neostigmine bromide is poorly absorbed from the gastrointestinal tract following oral administration
NEOSTIGMINE...IS ABSORBED POORLY AFTER ORAL ADMINISTRATION, SUCH THAT MUCH LARGER DOSES ARE NEEDED THAN BY THE PARENTERAL ROUTE. ...THE EFFECTIVE PARENTERAL DOSE OF NEOSTIGMINE IN MAN IS 0.5 TO 2.0 MG, THE EQUIVALENT ORAL DOSE MAY BE 30 MG OR MORE. LARGE ORAL DOSES MAY PROVE TOXIC IF INTESTINAL ABSORPTION IS ENHANCED FOR ANY REASON.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 108
...THE EXCRETION OF NEOSTIGMINE IS RETARDED IN PATIENTS WITH SEVERE KIDNEY DISEASE, MAKING THIS ANTICHOLINESTERASE DRUG AN ACCEPTABLE CHOICE IN PATIENTS WITH RENAL FAILURE.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 425
The pharmacokinetics of neostigmine in patients with normal renal function were determined and compared with those of patients undergoing renal transplantation or bilateral nephrectomy. Ten to 15 min prior to the end of operation and anesthesia, d-tubocurarine infusion was terminated and neostigmine, 0.07 mg/kg and atropine 0.03 mg/kg were given by infusion over a 2-min period. In anephric patients the elimination half-life was prolonged. Total serum clearance was decr from 16.7 ml/kg/min in patients with normal renal function to 7.8 ml/kg/min in anephric patients. Neostigmine pharmacokinetics following renal transplantation were not different from those in patients with normal renal function. Renal excretion accounts for 50% of neostigmine clearance.
Cronnelly R et al; Renal function and the pharmacokinetics of neostigmine in anesthetized man; Anesthesiology 51 (3): 222-6 (1979)
Neostigmine undergoes hydrolysis by cholinesterase and is also metabolized by microsomal enzymes in the liver.
NEOSTIGMINE IS DESTROYED BY PLASMA ESTERASES, AND THE QUATERNARY ALCOHOL AND PARENT COMPOUND ARE EXCRETED IN THE URINE.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 108
NEOSTIGMINE YIELDS 3-HYDROXYPHENYL TRIMETHYLAMMONIUM IN THE RAT. ROBERTS, JB ET AL; BIOCHEM PHARMAC 17: 9 (1968). /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. N-11
The half-life ranged from 42 to 60 minutes with a mean half-life of 52 minutes.
The pharmacokinetics of neostigmine was evaluated in man after iv and oral admin. The mean plasma T/2 for neostigmine after iv admin was 0.89 hr. Following oral admin peak concn occurred 1-2 hr after intake, but biol availability was only 1-2% of the admin dose. In patients with myasthenia gravis, the decrement of the evoked electric muscle response of repetitive nerve stimulation correlated well with plasma concn of neostigmine.
Eckernas SA et al; Pharmacokinetics of neostigmine and pyridostigmine in man and its correlation to clinical effects in myasthenia gravis; Adv Behav Biol 25 (Cholinergic Mech): 879-90 (1981)
Neostigmine is a parasympathomimetic, specifically, a reversible cholinesterase inhibitor. The drug inhibits acetylcholinesterase which is responsible for the degredation of acetylcholine. So, with acetylcholinesterase inhibited, more acetylcholine is present By interfering with the breakdown of acetylcholine, neostigmine indirectly stimulates both nicotinic and muscarinic receptors which are involved in muscle contraction.. It does not cross the blood-brain barrier.
...PHARMACOLOGICAL EFFECTS OF ANTICHOLINESTERASE AGENTS ARE DUE PRIMARILY TO PREVENTION OF HYDROLYSIS OF ACH /ACETYLCHOLINE/ BY ACHE /ACETYLCHOLINESTERASE/ AT SITES OF CHOLINERGIC TRANSMISSION. TRANSMITTER THUS ACCUMULATES, AND THE ACTION OF ACH /ACETYLCHOLINESTERASE/ THAT IS LIBERATED BY CHOLINERGIC IMPULSES OR THAT LEAKS FROM THE NERVE ENDING IS ENHANCED.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 103
Neostigmine increased both miniature end-plate potential and end-plate potential amplitudes but did not affect quantal content in isolated frog sciatic nerve-Sartorius muscle prepn. This suggests that cholinesterase inhibition was the only effect.
Alderdice MT; Physostigmine, but not neostigmine, inhibits acetylcholine release; Brain Res 178 (2-3): 596-9 (1979)
Long term (24-96 hr) treatment of a mouse-derived myogenic cell line (G8) with neostigmine markedly reduced binding of alpha-bungarotoxin (alpha-BuTx) to these cells. Protein synthesis in these cultures was markedly reduced and cell morphology degenerated. Myotubes maintained slightly hyperpolarized resting membrane potentials, and were able to respond to iontophoretic acetylcholine (Ach) application with overshooting action potentials. Degenerative changes at the neuromuscular junction associated with chronic neostigmine treatment in vivo are probably due to a direct action of the anticholinesterase on the muscle, rather than to altered intracleft ACh levels or to presynaptic effects of the anticholinesterase.
Noble MD et al; Direct effects of neostigmine on aneural myotube cultures; Neurosci Lett 11 (2): 149-54 (1979)
The intraluminal probe mounted with 2 electrode-strain gauge pairs, 4 cm apart, was used to study the effect of a neutral interview, a stressful interview, a meal (478.7 cal) and neostigmine (0.5 mg, im) on the contractile electrical complex, continuous electrical response activity and their associated contractions in 17 normal subjects. Neostigmine resulted in an incr in contractile electric complex & continuous electric response activity indexes 5-10 and 25-30 min after the injection, respectively. Both the meal and neostigmine incr the percentage of propagated contractile electric complexes during all of the recording periods.
Sarna S et al; The effects of stress, meal, and neostigmine on rectosigmoidal motility in normals; Motil Dig Tract (Proc Int Symp Gastrointest Motil) 8th: 499-511 (1982)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
43
PharmaCompass offers a list of Neostigmine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Neostigmine manufacturer or Neostigmine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Neostigmine manufacturer or Neostigmine supplier.
PharmaCompass also assists you with knowing the Neostigmine API Price utilized in the formulation of products. Neostigmine API Price is not always fixed or binding as the Neostigmine Price is obtained through a variety of data sources. The Neostigmine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Neostigmine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Neostigmine, including repackagers and relabelers. The FDA regulates Neostigmine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Neostigmine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Neostigmine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Neostigmine supplier is an individual or a company that provides Neostigmine active pharmaceutical ingredient (API) or Neostigmine finished formulations upon request. The Neostigmine suppliers may include Neostigmine API manufacturers, exporters, distributors and traders.
click here to find a list of Neostigmine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Neostigmine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Neostigmine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Neostigmine GMP manufacturer or Neostigmine GMP API supplier for your needs.
A Neostigmine CoA (Certificate of Analysis) is a formal document that attests to Neostigmine's compliance with Neostigmine specifications and serves as a tool for batch-level quality control.
Neostigmine CoA mostly includes findings from lab analyses of a specific batch. For each Neostigmine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Neostigmine may be tested according to a variety of international standards, such as European Pharmacopoeia (Neostigmine EP), Neostigmine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Neostigmine USP).
