
Synopsis
Synopsis
0
KDMF
0
VMF
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Bi Rg 587
2. Bi-rg-587
3. Birg587
4. Hemihydrate, Nevirapine
5. Nevirapine Hemihydrate
6. Viramune
1. 129618-40-2
2. Viramune
3. Bi-rg-587
4. Nevirapine Anhydrous
5. Birg 0587
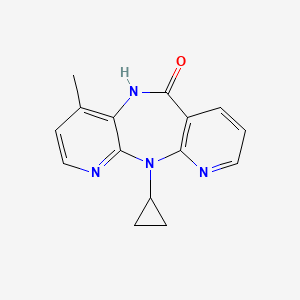
6. 11-cyclopropyl-4-methyl-5,11-dihydro-6h-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one
7. Nvp
8. Viramune Xr
9. Bi-rg 587
10. 11-cyclopropyl-5,11-dihydro-4-methyl-6h-dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one
11. Nevirapine, Anhydrous
12. 11-cyclopropyl-5,11-dihydro-4-methyl-6h-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one
13. Nevirapine Teva
14. Birg-0587
15. Nsc 641530
16. Nevirapine (viramune)
17. Chembl57
18. 11-cyclopropyl-4-methyl-5,11-dihydro-6h-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one
19. 11-cyclopropyl-4-methyl-5h-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6(11h)-one
20. Mfcd00866928
21. Nev
22. Nsc-641530
23. 99dk7fvk1h
24. 6h-dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-
25. Mls000084585
26. Chebi:63613
27. Nsc641530
28. Ncgc00065890-02
29. Smr000048458
30. Dsstox_cid_10787
31. Dsstox_rid_78889
32. Dsstox_gsid_31797
33. 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3,5,7,12,14-hexaen-10-one
34. Birg587
35. Viramune(tm)
36. Viramune (tn)
37. Cas-129618-40-2
38. Hsdb 7164
39. Nevirapine & Cd4-igg
40. Nevirapine & Pro 140
41. Unii-99dk7fvk1h
42. Nevirapine (jan/usp/inn)
43. Nevirapine)
44. 11-cyclopropyl-4-methyl-5h-dipyrido[[?],[?]][1,4]diazepin-6-one
45. Birg 587
46. Birg-587
47. Non-nucleoside Rt Inhibitor Nevirapine
48. 11-cyclopropyl-4-methyl-5h-dipyrido[2,3-b:3',2'-e][1,4]diazepin-6-one
49. Viramune Ir
50. 1vrt
51. 2hny
52. Nevirapine,(s)
53. Nevirapine [usan:usp:inn:ban]
54. Bi-rg-587 & Cd4-igg
55. 6h-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-
56. Nevirapinum Anhydrous
57. Nevirapine (anhydrous)
58. Nevirapine [mi]
59. Opera_id_934
60. Nevirapine [inn]
61. Nevirapine [jan]
62. Nevirapine [hsdb]
63. Nevirapine [usan]
64. Nevirapine [vandf]
65. Nevirapine [mart.]
66. Schembl3318
67. Nevirapine [who-dd]
68. Mls000759409
69. Mls001055309
70. Mls001201730
71. Mls001424058
72. Mls006011423
73. Bidd:gt0326
74. Nevirapine [ema Epar]
75. Bdbm1434
76. Zinc4778
77. Dtxsid7031797
78. Nevirapine [orange Book]
79. Nevirapine [ep Monograph]
80. Nevirapine [usp Impurity]
81. Nevirapine For Peak Identification
82. Hms2051j09
83. Hms2231o23
84. Hms3264d21
85. Hms3371e03
86. Hms3393j09
87. Hms3655i08
88. Hms3715b10
89. Nevirapine [usp Monograph]
90. Pharmakon1600-01503842
91. Albb-027264
92. Bcp05587
93. Tox21 110982
94. Tox21_110982
95. Tox21_200770
96. Ac-643
97. Ac1280
98. Bbl010768
99. Nsc759902
100. Stk580320
101. Nevirapine Anhydrous [usp-rs]
102. Nevirapine Anhydrous [who-ip]
103. Akos005504351
104. Tox21_110982_1
105. Ab07544
106. Ccg-100939
107. Db00238
108. Ks-5019
109. Nc00189
110. Nsc-759902
111. Ncgc00065890-03
112. Ncgc00065890-04
113. Ncgc00065890-05
114. Ncgc00065890-07
115. Ncgc00065890-14
116. Ncgc00258324-01
117. 11-cyclopropyl-4-methyl-5,11-dihydro-6h-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one & Pro 140 (anti-ccr5 Monoclonal Antibody)
118. 2-cyclopropyl-7-methyl-2,4,9,15-tetrazatricyclo[9.4.0.03,8]pentadeca-1(11),3,5,7,12,14-hexaen-10-one
119. Hy-10570
120. N11-cyclopropyl-4-methyl-5,11-dihydro-6h-dipyrido[3,2-b:2',3'-e]-[1,4]diazepin-6-one & Cd4-immunoadhesin
121. Nevirapine 100 Microg/ml In Acetonitrile
122. Sy009679
123. Bi-rg 587;nsc 641530;nvp
124. Db-041930
125. Ft-0607215
126. Ft-0672686
127. N0922
128. Nevirapinum Anhydrous [who-ip Latin]
129. S1742
130. Sw197569-2
131. C07263
132. D00435
133. Ab00393001-13
134. Ab00393001-15
135. Ab00393001_16
136. Ab00393001_17
137. 618n402
138. Q263713
139. F2173-0607
140. Z1695906730
141. Bi-rg-587; Birg 0587; Birg587; Hsdb 7164; Nsc 641530; Nvp
142. Nevirapine (anhydrous), European Pharmacopoeia (ep) Reference Standard
143. Nevirapine Anhydrous, United States Pharmacopeia (usp) Reference Standard
144. Nevirapine Solution, 1.0 Mg/ml In Methanol, Certified Reference Material
145. Nevirapine, Pharmaceutical Secondary Standard; Certified Reference Material
146. 11-cyclopropyl-5,11-dihydro-4-methyl-6h-dipyrido-[3,2-b:2',3'-e][1,4]diazepin-6-one
147. 11-cyclopropyl-5,11-dihydro-4-methyl-6h-dipyrido[3,2-b :2',3'-e][1,4 ]diazepin-6-one
148. 11-cyclopropyl-5,11-dihydro-4-methyl-6h-dipyrido[3,2-b:2 ,3 -e][1,4]diazepin-6-one
149. 11-cyclopropyl-5,11-dihydro-4-methyl-6h-dipyrido[3,2-b:2', 3'-e][1,4]diazepin-6-one
150. 6h-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-
151. Nevirapine For Peak Identification, European Pharmacopoeia (ep) Reference Standard
152. 11-cyclopropyl-4-methyl-5,11-dihydro-6h-dipyrido[3,2-b:2 Inverted Exclamation Mark ,3 Inverted Exclamation Mark -e][1,4]diazepin-6-one
153. 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaen-10-one
| Molecular Weight | 266.30 g/mol |
|---|---|
| Molecular Formula | C15H14N4O |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 266.11676108 g/mol |
| Monoisotopic Mass | 266.11676108 g/mol |
| Topological Polar Surface Area | 58.1 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 397 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Nevirapine |
| PubMed Health | Nevirapine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRAMUNE XR is the brand name for nevirapine extended-release tablets. Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Nevirapine is structurally a member of th... |
| Active Ingredient | Nevirapine |
| Dosage Form | Tablet, extended release; Tablet, for suspension; Tablet; Suspension |
| Route | oral; Oral |
| Strength | 200mg; 50mg/5ml; 400mg; 100mg; 50mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Macleods Pharma; Apotex; Aurobindo; Hetero Labs Ltd Iii; Mylan Labs; Sciegen Pharms; Sandoz; Prinston; Cipla; Strides Pharma; Emcure Pharma; Micro Labs |
| 2 of 6 | |
|---|---|
| Drug Name | Viramune |
| PubMed Health | Nevirapine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRAMUNE XR is the brand name for nevirapine extended-release tablets. Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Nevirapine is structurally a member of th... |
| Active Ingredient | Nevirapine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 200mg; 50mg/5ml |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 3 of 6 | |
|---|---|
| Drug Name | Viramune xr |
| Active Ingredient | Nevirapine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg; 100mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 4 of 6 | |
|---|---|
| Drug Name | Nevirapine |
| PubMed Health | Nevirapine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRAMUNE XR is the brand name for nevirapine extended-release tablets. Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Nevirapine is structurally a member of th... |
| Active Ingredient | Nevirapine |
| Dosage Form | Tablet, extended release; Tablet, for suspension; Tablet; Suspension |
| Route | oral; Oral |
| Strength | 200mg; 50mg/5ml; 400mg; 100mg; 50mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Macleods Pharma; Apotex; Aurobindo; Hetero Labs Ltd Iii; Mylan Labs; Sciegen Pharms; Sandoz; Prinston; Cipla; Strides Pharma; Emcure Pharma; Micro Labs |
| 5 of 6 | |
|---|---|
| Drug Name | Viramune |
| PubMed Health | Nevirapine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIRAMUNE XR is the brand name for nevirapine extended-release tablets. Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Nevirapine is structurally a member of th... |
| Active Ingredient | Nevirapine |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 200mg; 50mg/5ml |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 6 of 6 | |
|---|---|
| Drug Name | Viramune xr |
| Active Ingredient | Nevirapine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg; 100mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
Nevirapine is indicated, in combination with other antiretroviral agents, for the treatment of HIV-1 infection. Drug-resistant HIV emerges rapidly and uniformly when nevirapine is administered as monotherapy. Therefore, nevirapine should always be administered in combination with at least two other antiretroviral agents when it is used for the treatment of HIV-1 infection. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1997
Nevirapine is indicated for the prevention of mother-to-child transmission of HIV-1 infection. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1997
Nevirapine has been evaluated in pregnant HIV-infected women. In a landmark study performed in Uganda, a single, oral intrapartum dose of nevirapine followed by a single dose to the newborn was superior to more complicated zidovudine therapy in preventing vertical transmission. of HIV. Only 13% of nevirapine-treated women transmitted HIV compared to 21.5% of zidovudine-treated women.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1361
Severe, life-threatening, and in some cases fatal hepatotoxicity, including fulminant and cholestatic hepatitis (e.g., transaminase elevations with or without hyperbilirubinemia, prolonged partial thromboplastin time, or eosinophilia), hepatic necrosis, and hepatic failure, have been reported in patients receiving nevirapine. Although clinical presentation varied, frequently occurring features included nonspecific prodromal signs and symptoms of fatigue, malaise, anorexia, nausea, jaundice, liver tenderness, and/or hepatomegaly, with or without initially abnormal serum transaminase concentrations; a diagnosis of hepatotoxicity should be considered even if liver function tests are initially normal or alternative diagnoses are possible. Manifestations progressed over several days to hepatic failure with transaminase elevation, with or without hyperbilirubinemia, prolonged partial thromboplastin time, and/or eosinophilia. ... If nevirapine is discontinued because of hepatitis, it should be permanently discontinued and not reinitiated.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 683
Hepatotoxicity has been also reported in a small number of individuals receiving nevirapine as part of a combination regimen for post-exposure prophylaxis of nosocomial or sexual HIV exposure.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1999
Serious hepatotoxicity has been reported in individuals not infected with HIV who received multiple doses of nevirapine as part of a 2- or 3-drug regimen for postexposure prophylaxis following occupational or nonoccupational exposure to HIV. Adverse hepatic effects in these individuals have included end-stage liver failure requiring transplantation, clinical hepatitis (e.g., jaundice, fever, nausea, vomiting, abdominal pain, and/or hepatomegaly), and elevated serum ALT and AST concentrations without clinical hepatitis. ...
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 683
Severe and life-threatening skin reactions (e.g., Stevens-Johnson syndrome; toxic epidermal necrolysis; hypersensitivity reactions characterized by rash, constitutional findings, and organ dysfunction), including some fatalities, have occurred in patients receiving nevirapine. ...
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 684
For more Drug Warnings (Complete) data for NEVIRAPINE (15 total), please visit the HSDB record page.
For use in combination with other antiretroviral drugs in the ongoing treatment of HIV-1 infection.
FDA Label
* Tablets and oral suspension:
Viramune is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1-infected adults, adolescents, and children of any age.
Most of the experience with Viramune is in combination with nucleoside reverse-transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after Viramune should be based on clinical experience and resistance testing.
* 50- and 100-mg prolonged-release tablets:
Viramune is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1-infected adolescents and children three years and above and able to swallow tablets.
Prolonged-release tablets are not suitable for the 14-day lead-in phase for patients starting nevirapine. Other nevirapine formulations, such as immediate-release tablets or oral suspension should be used.
Most of the experience with Viramune is in combination with nucleoside reverse-transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after Viramune should be based on clinical experience and resistance testing.
* 400-mg prolonged-release tablets:
Viramune is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1-infected adults, adolescents and children three years and above and able to swallow tablets.
Prolonged-release tablets are not suitable for the 14-day lead-in phase for patients starting nevirapine. Other nevirapine formulations, such as immediate-release tablets or oral suspension should be used.
Most of the experience with Viramune is in combination with nucleoside reverse-transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after Viramune should be based on clinical experience and resistance testing.
Nevirapine Teva is indicated in combination with other anti-retroviral medicinal products for the treatment of HIV 1 infected adults, adolescents, and children of any age.
Most of the experience with nevirapine is in combination with nucleoside reverse transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after nevirapine should be based on clinical experience and resistance testing.
Treatment of human immunodeficiency virus (HIV-1) infection
Nevirapine is a non-nucleoside reverse transcriptase inhibitor (nNRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). HIV-2 RT and eukaryotic DNA polymerases (such as human DNA polymerases alpha, beta, or sigma) are not inhibited by nevirapine. Nevirapine is, in general, only prescribed after the immune system has declined and infections have become evident. It is always taken with at least one other HIV medication such as Retrovir or Videx. The virus can develop resistance to nevirapine if the drug is taken alone, although even if used properly, nevirapine is effective for only a limited time.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Cytochrome P-450 CYP3A Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inducers.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AG01
J05AG01
J05AG01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AG - Non-nucleoside reverse transcriptase inhibitors
J05AG01 - Nevirapine
Absorption
Nevirapine is readily absorbed (greater than 90%) after oral administration in healthy subjects and adults with HIV-1 infection. The absolute bioavailability in healthy adults following a single dose administration is 93 9% (mean SD) for a 50 mg tablet and 91 8% for an oral solution. Peak plasma nevirapine concentrations of 2 0.4 mcg/mL (7.5 micromolar) were attained by 4 hours following a single 200 mg dose. Nevirapine tablets and suspension have been shown to be comparably bioavailable and interchangeable at doses up to 200 mg. When the oral tablet is given with a high-fat meal, the extent of absorption is compared to that of the fasted-state.
Route of Elimination
Thus cytochrome P450 metabolism, glucuronide conjugation, and urinary excretion of glucuronidated metabolites represent the primary route of nevirapine biotransformation and elimination in humans. Only a small fraction (<5%) of the radioactivity in urine (representing <3% of the total dose) was made up of parent compound; therefore, renal excretion plays a minor role in elimination of the parent compound.
Volume of Distribution
1.21 0.09 L/kg [apparent volume of distribution, healthy adults, IV] Nevirapine is capable of crossing the placenta and is found in breast milk.
Nevirapine readily crosses the placenta and has been found in breast milk. The CSF-plasma ratio for nevirapine is approximately 0.45.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1360
Nevirapine is readily (more than 90%) absorbed following oral administration in healthy or HIV-infected adults. Absolute bioavailability of nevirapine in 12 healthy adults was 93% following administration of a single 30 mg tablet or 91% following administration of an oral solution of the drug. Peak plasma nevirapine concentrations average 2 ug/ml and are attained within 4 hours after a single 200 mg dose of the dose in adults. Following multiple doses, peak plasma nevirapine concentrations appear to increase linearly in the dosage range of 200-400 mg daily. Nevirapine dosage of 400 mg daily resulted in steady-state trough plasma concentrations of 4.5 ug/ml.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 687
Widely distributed. Nevirapine is highly lipophilic and essentially is nonionized at physiologic pH. Nevirapine readily crosses the placenta and is distributed into breast milk. Nevirapine concentrations in the cerebrospinal fluid were 45% of the concentrations in plasma, which is a ratio that is approximately equal to the fraction not bound to plasma protein.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1998
Nevirapine crosses the placenta in humans. In a limited number of HIV-infected pregnant women who received a single 100- or 200-mg oral dose of nevirapine 0.9-10.5 hours prior to delivery, cord blood concentrations of nevirapine were 74-123% of maternal serum concentrations and peak serum concentrations of the drug in the neonates of these women averaged 862 ng/mL (range: 257-1031 ng/mL) or 925 ng/mL (range: 62-2030 ng/mL), respectively. In pregnant HIV-infected women who received a regimen of nevirapine (200 mg once daily for 2 weeks followed by 200 mg twice daily), zidovudine, and lamivudine during the second and third trimester, cord blood concentrations (at delivery) and neonatal serum concentrations (24 hours after birth) of nevirapine were 76 and 60%, respectively, of maternal serum concentrations (at delivery). These neonatal concentrations were lower than those reported in neonates whose mothers received a single nevirapine dose during labor, possibly as the result of hepatic enzyme induction in the neonate after placental transfer.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 687
Nevirapine is distributed into human milk. Following administration of a single 100- or 200-mg dose of nevirapine to pregnant women several hours before delivery, postpartum, concentrations of the drug in milk were 25-122% of maternal serum concentrations.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 687
Hepatic. In vivo studies in humans and in vitro studies with human liver microsomes have shown that nevirapine is extensively biotransformed via cytochrome P450 3A4 metabolism to several hydroxylated metabolites.
Oxidative metabolism of nevirapine in the liver by cytochrome p450 isoforms CYP34A4 and CYP2B6 produces several metabolites including 2-, 3-, 8-, and 12-hydroxynevirapine.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1360
In vivo studies in humans and in vitro studies with human liver microsomes have shown that nevirapine is extensively biotransformed via cytochrome p450 oxidative metabolism to several hydroxylated metabolites. In vitro studies with human liver microsomes suggest that oxidative metabolism of nevirapine is mediated primarily by cytochrome p450 isozymes from the CYP3A family, although other isozymes may have a secondary role.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1998
Nevirapine has known human metabolites that include 12-hydroxy-nevirapine, 2-Hydroxynevirapine, 3-Hydroxynevirapine, and 8-Hydroxynevirapine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
45 hours
Approximately 45 hours after a single dose, and 25 to 30 hours following multiple dosing with 200 to 400 mg per day.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1998
Nevirapine binds directly to reverse transcriptase (RT) and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. The activity of nevirapine does not compete with template or nucleoside triphosphates.
The binding site for nevirapine on HIV-1 reverse transcriptase is near, but not at the proposed site of active polymerization, in a deep pocket lying between the beta sheets of the palm and at the base of the thumb subdomains of the enzyme's p66 subunit. In the absence of nevirapine, the binding of deoxynucleoside triphosphate to the reverse transcriptase-template complex results in a change in the conformation of reverse transcriptase. This conformational change is followed by a magnesium-dependent chemical reaction in which deoxynucleoside triphosphate is incorporated into the newly forming viral DNA; the conformational change appears to be the rate-limiting step of the reverse transcriptase catalysis of viral DNA formation. Nevirapine appears to have no appreciable effect on the rate of or equilibrium constant for the conformational change but may slow the chemical reaction, which then becomes the rate-limiting step in the catalytic sequence. When nevirapine binds to the reverse transcriptase-template complex, changes may occur in the position of aspartic acid carboxyl groups in reverse transcriptase so that magnesium ions are not in proper alignment for the chemical reaction to occur efficiently, and the reaction is slowed. Therefore, although the nevirapine-reverse transcriptase-template complex may continue to bind deoxynucleoside triphosphate and to catalyze its incorporation into the newly forming viral DNA, it appears to do so at a slower rate.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 686
The mechanism of action of nevirapine differs from that of nucleoside reverse transcriptase inhibitors (e.g., abacavir, didanosine, lamivudine, stavudine, zalcitabine, zidovudine). Nucleoside antiretroviral agents require intracellular conversion to triphosphate metabolites, which then compete with naturally occurring deoxynucleoside triphosphates for incorporation into viral DNA by reverse transcriptase and cause premature viral DNA chain termination by preventing further 5 to 3 phosphodiester linkages. Nevirapine, however, is noncompetitive with respect to primer-template or nucleoside triphosphate binding and is specific for HIV-1 reverse transcriptase. The drug binds directly to heterodimeric HIV-1 reverse transcriptase and appears to inhibit viral RNA- and DNA-dependent DNA polymerase activities by disrupting the catalytic site of the enzyme.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 686
Nevirapine diffuses into the cell and binds to reverse transcriptase adjacent to the catalytic site. This induces conformational changes that inactivate the enzyme. Resistance develops rapidly in cells exposed to nevirapine. High-level resistance is associated with mutations at reverse transcriptase codons 101, 103, 106,108, 135, 181, 188, and 190. A single mutation at either codon 103 or 181 decreases susceptibility more than 100 fold. Cross-resistance may extend to all FDA-approved nonnucleoside reverse transcriptase inhibitors, especially with the codon 103 mutation.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1360
Nevirapine is a highly specific inhibitor of HIV-1 reverse transcriptase, and results of in vitro studies indicate that nevirapine does not appear to inhibit cellular DNA polymerases, including human alpha-, beta-, Gamma-, or Delta-polymerases.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 686

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
93
PharmaCompass offers a list of Nevirapine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nevirapine manufacturer or Nevirapine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nevirapine manufacturer or Nevirapine supplier.
PharmaCompass also assists you with knowing the Nevirapine API Price utilized in the formulation of products. Nevirapine API Price is not always fixed or binding as the Nevirapine Price is obtained through a variety of data sources. The Nevirapine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nevirapine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nevirapine, including repackagers and relabelers. The FDA regulates Nevirapine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nevirapine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nevirapine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nevirapine supplier is an individual or a company that provides Nevirapine active pharmaceutical ingredient (API) or Nevirapine finished formulations upon request. The Nevirapine suppliers may include Nevirapine API manufacturers, exporters, distributors and traders.
click here to find a list of Nevirapine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nevirapine DMF (Drug Master File) is a document detailing the whole manufacturing process of Nevirapine active pharmaceutical ingredient (API) in detail. Different forms of Nevirapine DMFs exist exist since differing nations have different regulations, such as Nevirapine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nevirapine DMF submitted to regulatory agencies in the US is known as a USDMF. Nevirapine USDMF includes data on Nevirapine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nevirapine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nevirapine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nevirapine Drug Master File in Japan (Nevirapine JDMF) empowers Nevirapine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nevirapine JDMF during the approval evaluation for pharmaceutical products. At the time of Nevirapine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nevirapine suppliers with JDMF on PharmaCompass.
A Nevirapine CEP of the European Pharmacopoeia monograph is often referred to as a Nevirapine Certificate of Suitability (COS). The purpose of a Nevirapine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Nevirapine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Nevirapine to their clients by showing that a Nevirapine CEP has been issued for it. The manufacturer submits a Nevirapine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Nevirapine CEP holder for the record. Additionally, the data presented in the Nevirapine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Nevirapine DMF.
A Nevirapine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Nevirapine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Nevirapine suppliers with CEP (COS) on PharmaCompass.
A Nevirapine written confirmation (Nevirapine WC) is an official document issued by a regulatory agency to a Nevirapine manufacturer, verifying that the manufacturing facility of a Nevirapine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Nevirapine APIs or Nevirapine finished pharmaceutical products to another nation, regulatory agencies frequently require a Nevirapine WC (written confirmation) as part of the regulatory process.
click here to find a list of Nevirapine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nevirapine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nevirapine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nevirapine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nevirapine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nevirapine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nevirapine suppliers with NDC on PharmaCompass.
Nevirapine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nevirapine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nevirapine GMP manufacturer or Nevirapine GMP API supplier for your needs.
A Nevirapine CoA (Certificate of Analysis) is a formal document that attests to Nevirapine's compliance with Nevirapine specifications and serves as a tool for batch-level quality control.
Nevirapine CoA mostly includes findings from lab analyses of a specific batch. For each Nevirapine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nevirapine may be tested according to a variety of international standards, such as European Pharmacopoeia (Nevirapine EP), Nevirapine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nevirapine USP).
