
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
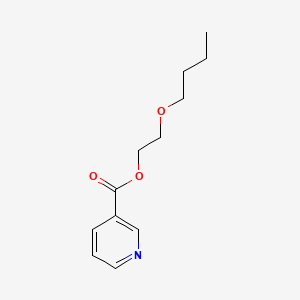

1. 2-butoxyethyl Nicotinate
2. Beta-butoxyethyl Nicotinate
1. Butoxyethyl Nicotinate
2. 13912-80-6
3. Beta-butoxyethyl Nicotinate
4. 2-butoxyethyl Pyridine-3-carboxylate
5. 2-butoxyethyl Nicotinate
6. 1322-29-8
7. Nicoboxil [inn]
8. 3-pyridinecarboxylic Acid, 2-butoxyethyl Ester
9. Gsd5b9us0w
10. Chebi:32322
11. Nicoboxil (inn)
12. Nicoboxilo
13. Nicoboxilum
14. Beta-butoxyethyl Nicotinate (jan)
15. Beta-butoxyethyl Nicotinate [jan]
16. Sr-01000945168
17. Nicoboxilum [inn-latin]
18. Unii-gsd5b9us0w
19. Nicoboxilo [inn-spanish]
20. Butoxyethyl 3-pyridinecarboxylate
21. Nicotinsaeure-beta-butoxyethylester
22. Einecs 237-684-7
23. 3-pyridinecarboxylic Acid, Butoxyethyl Ester
24. Nicoboxil [mart.]
25. Nicoboxil [who-dd]
26. Dsstox_cid_31424
27. Dsstox_rid_97310
28. Dsstox_gsid_57635
29. Schembl288321
30. Chembl2105161
31. Dtxsid4057635
32. 2-butoxyethyl 3-pyridinecarboxylate
33. Zinc2020020
34. Tox21_113682
35. .beta.-butoxyethyl Nicotinate
36. Butoxyethyl Nicotinate [inci]
37. Akos030543403
38. Db12911
39. Ncgc00249906-01
40. Cas-13912-80-6
41. Ft-0729684
42. D01677
43. 912n806
44. Sr-01000945168-1
45. Sr-01000945168-2
46. Q27114872
47. Z743362616
| Molecular Weight | 223.27 g/mol |
|---|---|
| Molecular Formula | C12H17NO3 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 223.12084340 g/mol |
| Monoisotopic Mass | 223.12084340 g/mol |
| Topological Polar Surface Area | 48.4 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 197 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The primary therapeutic use for which nicoboxil is currently indicated for is as an active ingredient in combination with the capsaicinoid nonivamide compound as a topical analgesic for the temporary relief of the pain of rheumatism, arthritis, lumbago, muscular aches, sprains and strains, sporting injuries, and other conditions where local warmth is beneficial. Nevertheless, most of the available studies regarding the use of nicoboxil and nonivamide topical analgesics focus specifically on their efficacy and safety in treating acute non-specific low back pain, typically finding the combination analgesic to be an effective, safe, and well-tolerated medication for such an indication.
Topical applications consisting of the individual active ingredients of nicoboxil and nonivamide at doses considered to be therapeutic are generally not considered readily available commercially. Subsequently, the pharmacodynamics of nicoboxil are considered useful in commercially available combination products largely because they combine with those of nonivamide to offer a synergistic effect from the unique complementary actions of these two agents. Subsequently, nonivamide is a synthetic capsaicin analog with analgesic properties which are assumed to result from the depletion of Substance P in the peripheral nociceptive C-fibres and A-delta nerve fibers upon repetitive topical application. Resultant stimulation of afferent nerve endings in the skin evidently causes a dilatory effect on the surrounding blood vessels accompanied by an intense, long-lasting sensation of warmth associated with the nonivamide use. Given the proposed effect of nonivamide, it is believed that nicoboxil is a vitamin of the B complex that possesses vasodilating properties facilitated by prostaglandin. The observed hyperaemic increased blood flow effect of nicoboxil occurs earlier and is described as being more intense than the nonivamide hyperaemic effect. Nicoboxil and nonivamide are consequently generally indicated as a combination product because the pharmacodynamics of nicoboxil are considered useful as a complement to those of nonivamide, and vice versa. In essence, both compounds induce vasodilation by different effects and therefore have complementary abilities inducing increased blood flow, thus hastening the hyperaemic skin reaction.
Absorption
Specific investigations on absorption of dermally applied nicoboxil in laboratory animals or target species were not available. Published data for nicotinate esters related to nicoboxil indicated however, that members of this class of compounds are in principle able to penetrate skin [12]. Regardless, there is interest in the studies that demonstrate nicoboxil and nonivamide combination topical applications as effective and safe analgesic products precisely because such topical formulations are expected to have much lower systemic absorption - and thus less exposure to systemic side effects (ie. like gastrointestinal upset, drowsiness, etc.) - than the oral non-steroidal anti-inflammatory drugs, opioids, muscle relaxants, and steroids that may be more commonly prescribed over a rubefacient like nicoboxil. Nevertheless, despite the fact that topical nicoboxil and nonivamide products been available to use in some parts of Europe since the 1950s to treat discomfort of the muscuoskeletal system, the effects of nicoboxil and nonivamide have not been investigated in detail and a lack of detailed studies on nicoboxil pharmacodynamics and pharmacokinetics remains ongoing.
Route of Elimination
Following ester cleavage, the nicotinic acid metabolite is expected to enter the endogenous metabolic pool as a part of the vitamin B complex. The 2-butoxyethanol metabolite is believed to be mainly excreted primarily in the urine and to a certain extent, in exhaled air. In humans, the urinary elimination of 2-butoxyethanol's metabolite, 2-butoxyacetic acid was also reported.
Volume of Distribution
Despite the fact that topical nicoboxil and nonivamide products been available to use in some parts of Europe since the 1950s to treat discomfort of the muscuoskeletal system, the effects of nicoboxil and nonivamide have not been investigated in detail and a lack of detailed studies on nicoboxil pharmacodynamics and pharmacokinetics remains ongoing. Readily accessible data regarding the volume of distribution of nicoboxil is subsequently not available.
Clearance
The elimination of nicoboxil is considered to be rapid. Despite the fact that topical nicoboxil and nonivamide products been available to use in some parts of Europe since the 1950s to treat discomfort of the muscuoskeletal system, the effects of nicoboxil and nonivamide have not been investigated in detail and a lack of detailed studies on nicoboxil pharmacodynamics and pharmacokinetics remains ongoing. Readily accessible data regarding the clearance of nicoboxil is subsequently not available.
Any systemically absorbed nicoboxil is expected to be hydrolyzed to nicotinic acid and 2-butoxyethanol in blood plasma. In vitro it is reported that such hydrolysis reactions are catalyzed by esterase-like activity of serum albumin and by plasma esterases. The nicotinic acid metabolite is also capable of vascular dilatation. In humans, the urinary elimination of 2-butoxyethanol's metabolite, 2-butoxyacetic acid was also reported. The metabolism of nicoboxil is considered to be rapid.
The half-life of ester hydrolysis was found to be very short in the presence of human serum albumin - less than 15 minutes, 50uM.
In particular, nicoboxil is considered a rubefacient. However, the specific mechanism of action by which rubefacients like nicoboxil elicit pharmacologic effects has not yet been formally elucidated. Nevertheless, it is generally proposed that rubefacients cause irritation of the skin when applied topically, and are believed to alleviate pain in muscles, joints, tendons, and other musculoskeletal pains in the extremities by counter-irritation. This specific term, 'counter-irritant', derives from the fact that rubefacients can cause a reddening of the skin by causing the blood vessels of the skin to dilate, which gives a soothing feeling of warmth. In essence, the term largely refers to the notion that irritation of the sensory nerve endings alters or offsets pain in the underlying muscle or joints that are innervated by the same nerves. In fact, the vasodilation effect of rubefacients like nicoboxil has been considered the result of nerve conduction mechanisms as early as the late 1950s when certain studies demonstrated that the concomitant application of xylocaine could counteract or prevent the vasolidator response to rubefacients in 50% of such related experiments.
Global Sales Information

Market Place

ABOUT THIS PAGE
13
PharmaCompass offers a list of Nicoboxil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nicoboxil manufacturer or Nicoboxil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nicoboxil manufacturer or Nicoboxil supplier.
PharmaCompass also assists you with knowing the Nicoboxil API Price utilized in the formulation of products. Nicoboxil API Price is not always fixed or binding as the Nicoboxil Price is obtained through a variety of data sources. The Nicoboxil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nicoboxil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nicoboxil, including repackagers and relabelers. The FDA regulates Nicoboxil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nicoboxil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Nicoboxil supplier is an individual or a company that provides Nicoboxil active pharmaceutical ingredient (API) or Nicoboxil finished formulations upon request. The Nicoboxil suppliers may include Nicoboxil API manufacturers, exporters, distributors and traders.
Nicoboxil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nicoboxil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nicoboxil GMP manufacturer or Nicoboxil GMP API supplier for your needs.
A Nicoboxil CoA (Certificate of Analysis) is a formal document that attests to Nicoboxil's compliance with Nicoboxil specifications and serves as a tool for batch-level quality control.
Nicoboxil CoA mostly includes findings from lab analyses of a specific batch. For each Nicoboxil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nicoboxil may be tested according to a variety of international standards, such as European Pharmacopoeia (Nicoboxil EP), Nicoboxil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nicoboxil USP).
