
Synopsis
Synopsis
0
KDMF
0
VMF
0
Canada

Annual Reports
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
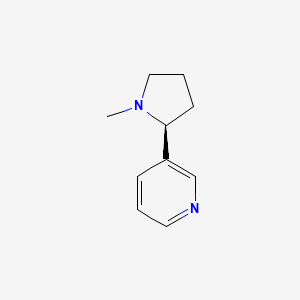

1. Nicotine Bitartrate
2. Nicotine Tartrate
1. L-nicotine
2. 54-11-5
3. (-)-nicotine
4. (s)-nicotine
5. (s)-3-(1-methylpyrrolidin-2-yl)pyridine
6. Habitrol
7. (s)-(-)-nicotine
8. Nicotrol
9. 3-[(2s)-1-methylpyrrolidin-2-yl]pyridine
10. Nicoderm
11. Nicoderm Cq
12. Fumetobac
13. Prostep
14. Nicotine Polacrilex
15. Flux Maag
16. Ortho N-4 Dust
17. Ortho N-5 Dust
18. Xl All Insecticide
19. Niagara P.a. Dust
20. Nicorette
21. Nicotina
22. Nikotyna
23. Destruxol Orchid Spray
24. 3-(n-methylpyrollidino)pyridine
25. 3-(n-methylpyrrolidino)pyridine
26. L-3-(1-methyl-2-pyrrolidyl)pyridine
27. L(-)-nicotine
28. (-)-3-(1-methyl-2-pyrrolidyl)pyridine
29. Ortho N-4 And N-5 Dusts
30. Nicocide
31. Nicotin
32. Tendust
33. Nico-dust
34. Emo-nik
35. Nico-fume
36. Nicotine Alkaloid
37. Mach-nic
38. Nic-sal
39. Nsc 5065
40. Beta-pyridyl-alpha-n-methylpyrrolidine
41. 3-(2-(n-methylpyrrolidinyl))pyridine
42. Pyridine, 3-(1-methyl-2-pyrrolidinyl)-, (s)-
43. 3-((2s)-1-methylpyrrolidin-2-yl)pyridine
44. 3-(1-methyl-2-pyrollidinyl)pyridine
45. (-)-3-(n-methylpyrrolidino)pyridine
46. S-(-)-nicotine
47. (s)-3-(1-methyl-2-pyrrolidinyl)pyridine
48. 3-(1-methyl-2-pyrrolidinyl)pyridine
49. (s)-3-(n-methylpyrrolidin-2-yl)pyridine
50. 6m3c89zy6r
51. Micotine
52. Chebi:17688
53. Pyridine, 3-((2s)-1-methyl-2-pyrrolidinyl)-
54. Pyridine, 3-[(2s)-1-methyl-2-pyrrolidinyl]-
55. 1-methyl-2-(3-pyridiyl)pyrrolidine
56. Nikotin [german]
57. Nikotyna [polish]
58. Nicotina [italian]
59. Dsstox_cid_930
60. Nicotrol Inhaler
61. Nicotrol Ns
62. Nicotine [for Single Use]
63. Nicotine-l (base)
64. 104062-50-2
65. Destruxol
66. Dsstox_rid_75874
67. Nicotine And Salts
68. Dsstox_gsid_20930
69. Pyrrolidine, 1-methyl-2-(3-pyridal)-
70. (-)-1-methyl-2-(3-pyridyl)pyrrolidine
71. Fumeto Bac
72. Caswell No. 597
73. Pyridine, 3-(tetrahydro-1-methylpyrrol-2-yl)
74. Nicabate
75. Nicotine [bsi:iso]
76. Nicotinum
77. Niquitin
78. Tabazur
79. Exodus
80. Nicotine-d Salicylate
81. Rcra Waste Number P075
82. Cas-54-11-5
83. Dl-tetrahydronicotyrine
84. Ent 3,424
85. Habitrol (tn)
86. Nicotine (usp)
87. Nicotine [iso]
88. Smr000059074
89. Ccris 1637
90. Nicotine (compounds Related To)
91. Hsdb 1107
92. Nicotine [usp:ban]
93. Einecs 200-193-3
94. Methyl-2-pyrrolidinyl)pyridine
95. Mfcd00006369
96. Un1654
97. Rcra Waste No. P075
98. Epa Pesticide Chemical Code 056702
99. Beta-pyridyl-alpha-n-methyl Pyrrolidine
100. Unii-6m3c89zy6r
101. Delta-nicotine
102. Nsc-5065
103. Ai3-03424
104. Nicotine Betadex
105. 1-methyl-2-(3-pyridal)-pyrrolidene
106. Nicotine S(-)
107. A-n-methylpyrrolidine
108. Campbell's Nico-soap
109. 3-n-methylpyrrolidine
110. R)-(+)-nicotine
111. A -n-methylpyrrolidine
112. Chembl3
113. 1uw6
114. Nicotine [hsdb]
115. Alpha-n-methylpyrrolidine
116. Nicotine [mi]
117. Nicotine [un1654]
118. Nicotine [vandf]
119. Nicotinum [hpus]
120. (s)-(-)--nicotine
121. Nicotine [mart.]
122. Bmse000105
123. Nicotine [who-dd]
124. (2s) 3-(1-methyl-pyrrolidin-2-yl)-pyridine
125. Ec 200-193-3
126. Nicotine Polacrilex [usan]
127. S-()-nicotine-pyridine-d4
128. Schembl20192
129. Mls001055457
130. Mls001335905
131. Bidd:gt0599
132. Nicotine [orange Book]
133. Gtpl2585
134. Nicotine [ep Monograph]
135. Dtxsid1020930
136. Nicotine [usp Monograph]
137. Bdbm82070
138. Hms2230h17
139. Hms3259e16
140. Nicotine [un1654] [poison]
141. Zinc391812
142. Hy-b0638
143. Nicotine 10 Microg/ml In Methanol
144. Nicotine Component Of Commit
145. Tox21_201814
146. Tox21_300174
147. Nicotine 100 Microg/ml In Methanol
148. Pdsp1_000113
149. Pdsp1_000465
150. Pdsp2_000463
151. Pdsp2_000555
152. 1-methyl-2-(3-pyridal)-pyrrolidine
153. Akos016843798
154. Nicotine 1000 Microg/ml In Methanol
155. Nicotine Component Of Nicorette
156. 3-(1-methyl-2-pyrrolidinyl)-pyridine
157. Ccg-204892
158. Cs-3999
159. Db00184
160. Nc00577
161. Sb12751
162. Sdccgmls-0066911.p001
163. Sdccgsbi-0050785.p004
164. Ncgc00090693-01
165. Ncgc00090693-02
166. Ncgc00090693-03
167. Ncgc00090693-04
168. Ncgc00090693-05
169. Ncgc00090693-06
170. Ncgc00090693-07
171. Ncgc00090693-08
172. Ncgc00090693-09
173. Ncgc00254095-01
174. Ncgc00259363-01
175. Pyrrolidine, 1-methyl-2-(3-pyridyl)-
176. (-)-nicotine, >=99% (gc), Liquid
177. Sbi-0050785.p003
178. Cas_29790-52-1
179. N0079
180. C00745
181. D03365
182. P10017
183. (-)-.beta.-pyridyl-.alpha.-n-methylpyrrolidine
184. Ab00694322_12
185. (-)-nicotine, Pestanal(r), Analytical Standard
186. 006n369
187. .beta.-pyridyl-.alpha.-n-methyl Pyrrolidine
188. 3-((2s)-1-methyl-2-pyrrolidinyl)pyridine
189. 3-[(1r)-1beta-methylpyrrolidine-2alpha-yl]pyridine
190. J-500021
191. Sr-05000001762-5
192. Brd-k05395900-322-02-1
193. Brd-k05395900-322-04-7
194. Pyridine, 3-(tetrahydro-1-methylpyrrol-2-yl), (s)-
195. Q28086552
196. Z1954805269
197. 434f7990-3240-4a43-acec-e6cc1e495fa0
198. (-)-nicotine Solution, 1.0 Mg/ml, Analytical Standard, For Drug Analysis
199. (s)-(-)-nicotine; 3-[(2s)-1-methyl-2-pyrrolidinyl] Pyridine
200. (-)-nicotine Solution, 100 Mug/ml In Acetonitrile, Pestanal(r), Analytical Standard
201. S(-)-nicotine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 162.23 g/mol |
|---|---|
| Molecular Formula | C10H14N2 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 162.115698455 g/mol |
| Monoisotopic Mass | 162.115698455 g/mol |
| Topological Polar Surface Area | 16.1 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 147 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Habitrol |
| PubMed Health | Nicotine |
| Drug Classes | Cholinergic, Smoking Cessation Agent |
| Active Ingredient | Nicotine |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 7mg/24hr; 21mg/24hr; 14mg/24hr |
| Market Status | Over the Counter |
| Company | Novartis |
| 2 of 10 | |
|---|---|
| Drug Name | Nicoderm cq |
| PubMed Health | Nicotine (Transdermal route) |
| Drug Classes | Cholinergic, Smoking Cessation Agent |
| Active Ingredient | Nicotine |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 7mg/24hr; 21mg/24hr; 14mg/24hr |
| Market Status | Over the Counter |
| Company | Sanofi Aventis Us |
| 3 of 10 | |
|---|---|
| Drug Name | Nicotine |
| PubMed Health | Nicotine |
| Drug Classes | Cholinergic, Smoking Cessation Agent |
| Drug Label | NICOTROL Inhaler (nicotine inhalation system) consists of a mouthpiece and a plastic cartridge delivering 4 mg of nicotine from a porous plug containing 10 mg nicotine. The cartridge is inserted into the mouthpiece prior to use. Nicotine is a terti... |
| Active Ingredient | Nicotine |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 7mg/24hr; 21mg/24hr; 14mg/24hr |
| Market Status | Over the Counter |
| Company | Aveva |
| 4 of 10 | |
|---|---|
| Drug Name | Nicotine polacrilex |
| PubMed Health | Nicotine (Into the nose) |
| Drug Classes | Smoking Cessation Agent |
| Active Ingredient | Nicotine polacrilex |
| Dosage Form | Troche/lozenge; Gum, chewing |
| Route | Buccal; Oral |
| Strength | eq 4mg base; eq 2mg base |
| Market Status | Over the Counter |
| Company | Perrigo R And D; Watson Labs; Ivax Sub Teva Pharms; Perrigo |
| 5 of 10 | |
|---|---|
| Drug Name | Nicotrol |
| Drug Label | NICOTROL Inhaler (nicotine inhalation system) consists of a mouthpiece and a plastic cartridge delivering 4 mg of nicotine from a porous plug containing 10 mg nicotine. The cartridge is inserted into the mouthpiece prior to use. Nicotine is a terti... |
| Active Ingredient | Nicotine |
| Dosage Form | Spray, metered; Inhalant |
| Route | Oral; Nasal |
| Strength | 0.5mg/spray; 4mg/cartridge |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn; Pfizer |
| 6 of 10 | |
|---|---|
| Drug Name | Habitrol |
| PubMed Health | Nicotine |
| Drug Classes | Cholinergic, Smoking Cessation Agent |
| Active Ingredient | Nicotine |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 7mg/24hr; 21mg/24hr; 14mg/24hr |
| Market Status | Over the Counter |
| Company | Novartis |
| 7 of 10 | |
|---|---|
| Drug Name | Nicoderm cq |
| PubMed Health | Nicotine (Transdermal route) |
| Drug Classes | Cholinergic, Smoking Cessation Agent |
| Active Ingredient | Nicotine |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 7mg/24hr; 21mg/24hr; 14mg/24hr |
| Market Status | Over the Counter |
| Company | Sanofi Aventis Us |
| 8 of 10 | |
|---|---|
| Drug Name | Nicotine |
| PubMed Health | Nicotine |
| Drug Classes | Cholinergic, Smoking Cessation Agent |
| Drug Label | NICOTROL Inhaler (nicotine inhalation system) consists of a mouthpiece and a plastic cartridge delivering 4 mg of nicotine from a porous plug containing 10 mg nicotine. The cartridge is inserted into the mouthpiece prior to use. Nicotine is a terti... |
| Active Ingredient | Nicotine |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 7mg/24hr; 21mg/24hr; 14mg/24hr |
| Market Status | Over the Counter |
| Company | Aveva |
| 9 of 10 | |
|---|---|
| Drug Name | Nicotine polacrilex |
| PubMed Health | Nicotine (Into the nose) |
| Drug Classes | Smoking Cessation Agent |
| Active Ingredient | Nicotine polacrilex |
| Dosage Form | Troche/lozenge; Gum, chewing |
| Route | Buccal; Oral |
| Strength | eq 4mg base; eq 2mg base |
| Market Status | Over the Counter |
| Company | Perrigo R And D; Watson Labs; Ivax Sub Teva Pharms; Perrigo |
| 10 of 10 | |
|---|---|
| Drug Name | Nicotrol |
| Drug Label | NICOTROL Inhaler (nicotine inhalation system) consists of a mouthpiece and a plastic cartridge delivering 4 mg of nicotine from a porous plug containing 10 mg nicotine. The cartridge is inserted into the mouthpiece prior to use. Nicotine is a terti... |
| Active Ingredient | Nicotine |
| Dosage Form | Spray, metered; Inhalant |
| Route | Oral; Nasal |
| Strength | 0.5mg/spray; 4mg/cartridge |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn; Pfizer |
Ganglionic Stimulants; Nicotinic Agonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Nicotine/ is indicated as an aid to smoking cessation for the relief of nicotine withdrawal symptoms. /Nicotine/ therapy should be used as a part of a comprehensive behavioral smoking cessation program. /Included in US product label/[US Natl Inst Health; DailyMed. Current Medication Information for Nicotrol
Nicotine] (August 2007). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=5011
In 2 children, use of nicotine polacrilex gum and haloperidol improved symptoms (eg, tics) of tourette's syndrome. /Not included in US product label/
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 1344
/EXPL THER/ Pyoderma gangrenosum is potentially a devastating and destructive disorder. There is no uniformly effective or specific therapy for pyoderma gangrenosum. Previous reports of nicotine therapy for pyoderma gangrenosum have suggested it to be efficacious. Unfortunately, previous reports were restricted by the use of commercially available preparations of nicotine, either as a gum or patch formulation. /The authors/ used topical nicotine 0.5% w/w cetamacrogol formula A cream that enables direct application onto the lesion, as well as dose and concentration variation. Two patients with pyoderma gangrenosum treated with topical nicotine 0.5% w/w cetamacrogol formula A cream are described here, both of whom had dramatic clinical resolution of their pyoderma gangrenosum.
PMID:15204166 Patel GK et al; J Dermatolog Treat 15 (2): 122-5 (2004)
For more Therapeutic Uses (Complete) data for NICOTINE (8 total), please visit the HSDB record page.
Drugs of Abuse: Contraindicated during Breast-Feeding: Nicotine (smoking): Shock, vomiting, diarrhea, rapid heart rate, restlessness; decreased milk production. (The Committee on Drugs strongly believes that nursing mothers should not ingest any compounds listed /drugs of abuse/ ... Not only are they hazardous to the nursing infant, but they are also detrimental to the physical and mental health of the mother ... No drug of abuse should be ingested by nursing mothers even though adverse reports are not in the literature.) /from Table 2/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 138 (1994)
Adverse local effects of nicotine polacrilex gum are related principally to the mechanical effects of gum chewing and include traumatic injury to the oral mucosa and/or teeth; irritation and/or tingling of the tongue, mouth, and throat; ulceration of the oral mucosa, including traumatic and aphthous ulcers; esophagitis; jaw-muscle ache; eructation (belching) resulting from unintentional swallowing of air during chewing; gum sticking to teeth; and unpleasant taste. A pruritic, maculopapular rash occurred periorally in at least one patient receiving nicotine polacrilex therapy. Some oral mucosal changes such as stomatitis, glossitis, gingivitis, pharyngitis, aphthous ulcers, and alterations in taste perception can occur during smoking cessation efforts with or without nicotine polacrilex therapy.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Unlike the adverse effect profile observed with buccal nicotine polacrilex gum where adverse local effects are most common, local effects occur less commonly than systemic effects with buccal lozenges of the drug. Adverse local effects reported with nicotine polacrilex lozenges in a placebo-controlled study were limited to sore throat, which occurred in 3.6% of patients receiving the drug.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Transient and localized pruritus, burning, or erythema on at least one occasion occurred in 35-54% of patients receiving transdermal nicotine therapy in controlled clinical trials. Pruritus or burning generally occurred just after application of a transdermal system and lasted no longer than 30 minutes. Local erythema, which generally resolved without treatment within 24 hours, was observed at least once following removal of a transdermal system in 7-25% of patients in clinical studies. Some degree of edema at the site of application was observed in 3-8% of patients using transdermal systems of nicotine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
For more Drug Warnings (Complete) data for NICOTINE (32 total), please visit the HSDB record page.
The fatal adult dose is 60 mg.
Zenz, C., O.B. Dickerson, E.P. Horvath. Occupational Medicine. 3rd ed. St. Louis, MO., 1994, p. 641
For the relief of nicotine withdrawal symptoms and as an aid to smoking cessation.
FDA Label
Nicotine, the primary alkaloid in tobacco products binds stereo-selectively to nicotinic-cholinergic receptors on autonomic ganglia, the adrenal medulla, neuromuscular junctions and in the brain. Nicotine exerts two effects, a stimulant effect exerted at the locus ceruleus and a reward effect in the limbic system. Itranvenous administration of nicotine causes release of acetylcholine, norepinephrine, dopamine, serotonine, vasopressin, beta-endorphin and ACTH. Nicotine is a highly addictive substance. Nicotine also induces peripheral vasoconstriction, tachycardia and elevated blood pressure. Nicotine inhalers and patches are used to treat smoking withdrawl syndrome. Nicotine is classified as a stimulant of autonomic ganglia.
Ganglionic Stimulants
Agents that mimic neural transmission by stimulation of the nicotinic receptors on postganglionic autonomic neurons. Drugs that indirectly augment ganglionic transmission by increasing the release or slowing the breakdown of acetylcholine or by non-nicotinic effects on postganglionic neurons are not included here nor are the nonspecific cholinergic agonists. (See all compounds classified as Ganglionic Stimulants.)
Nicotinic Agonists
Drugs that bind to and activate nicotinic cholinergic receptors (RECEPTORS, NICOTINIC). Nicotinic agonists act at postganglionic nicotinic receptors, at neuroeffector junctions in the peripheral nervous system, and at nicotinic receptors in the central nervous system. Agents that function as neuromuscular depolarizing blocking agents are included here because they activate nicotinic receptors, although they are used clinically to block nicotinic transmission. (See all compounds classified as Nicotinic Agonists.)
Smoking Cessation Agents
Substances that facilitate the cessation of tobacco smoking. (See all compounds classified as Smoking Cessation Agents.)
N07BA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N07 - Other nervous system drugs
N07B - Drugs used in addictive disorders
N07BA - Drugs used in nicotine dependence
N07BA01 - Nicotine
Absorption
Absorption of nicotine through the buccal mucosa is relatively slow and the high and rapid rise followed by the decline in nicotine arterial plasma concentrations seen with cigarette smoking are not achieved with the inhaler. About 10% of absorbed nicotine is excreted unchanged in urine.
Route of Elimination
About 10% of the nicotine absorbed is excreted unchanged in the urine.
Volume of Distribution
2 to 3 L/kg
Clearance
1.2 L/min [healthy adult smoker]
Nicotine is readily absorbed from respiratory tract, buccal mucous membranes, and skin. ... Both nicotine and its metabolites are rapidly eliminated by the kidneys. The rate of urinary excretion of nicotine is dependent upon pH of urine; excretion diminishes when urine is alkaline. Nicotine is also excreted in milk of lactating women who smoke. Milk of heavy smokers may contain 0.5 mg/L. ...Apparently the gastric absorption of nicotine from tobacco taken by mouth is delayed because of slowed gastric emptying, so that vomiting may remove much of the tobacco remaining in GI tract.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 192
Although nicotine is absorbed rapidly over large section of GI tract, absorption of n-oxide is limited to area relatively high in intestine. n-Oxide is reduced to nicotine in gut and nicotine produced is absorbed low enough in GI tract to avoid first pass phenomenon.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 156
Free alkaloid is absorbed rapidly through skin and gastrointestinal and respiratory tracts, but absorption of its acid salts is less complete.
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 3378
The pharmacokinetics of various commercially available dosage forms of nicotine and nicotine polacrilex differ principally in the rate, site, and extent of absorption of the drug, with absorption being most rapid with intranasal administration of the spray (peak concentrations achieved within 4-15 minutes), followed by chewing the gum (peaks within 25-30 minutes) or oral inhalation (peaks within 15-30 minutes), and then being substantially slower with the transdermal systems (peaks within 2-10 hours). Plasma nicotine concentrations fluctuate least with the transdermal systems and are least like those produced by cigarette smoking, whereas those produced by intranasal administration mimic those of cigarette smoking most closely, although the role of their rise is still somewhat slower and peaks achieved generally are lower than with cigarettes.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
For more Absorption, Distribution and Excretion (Complete) data for NICOTINE (16 total), please visit the HSDB record page.
Primarily hepatic, cotinine is the primary metabolite.
Metabolism of nicotine is qualitatively similar following buccal absorption from nicotine polacrilex gum or nicotine oral inhalation and from oral inhalation of cigarette smoke, and reportedly following application of nicotine transdermal systems. Although the exact metabolic fate of nicotine is not clearly established, the drug is metabolized extensively to more than 20 metabolites, but principally in the liver via oxidation of the alpha-carbon and N-oxidation of the pyrrolidine ring to cotinine and nicotine-1'-N-oxide, respectively. These metabolites are not pharmacologically active in humans at blood concentrations attained during cigarette smoking; however, cotinine has been reported to be pharmacologically active in animals, but its potency at equivalent molar concentrations is substantially less than that of nicotine. ... Nicotine may also be metabolized to a lesser extent in the kidneys and lungs.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Nicotine-1'-N-oxide is reduced to nicotine by bacterial flora in the large intestine via an N-oxide reductase system and subsequently undergoes enterohepatic circulation and repeat metabolism in the liver.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Toxicologically, it is of interest that /the microsomal flavin-containing monooxygenase/ is responsible for the oxidation of nicotine to nicotine-1'-N-oxide, whereas the oxidation of nicotine to cotinine is catalyzed by two enzymes acting in sequence: P450 and a soluble aldehyde dehydrogenase. Thus, nicotine is metabolized by two different routes, the relative contributions of which may vary with both the extrinsic and intrinsic factors.
Hodgson E, Levi PE; A Textbook of Modern Toxicology 2nd ed p.74 (1997)
In vitro studies with rabbit liver microsomes, /NADPH/, and O2, indicated that metabolism of nicotine proceeded through hydroxylation to 5-(3'-pyridyl)-n-methylpyrrolidine-2-ol; oxidation to cotinine; and deamidation of cotinine to 4-(3'-pyridyl)-4-methylamino-butyric acid. No carbon dioxide was observed.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 258
For more Metabolism/Metabolites (Complete) data for NICOTINE (11 total), please visit the HSDB record page.
Nicotine has known thirdhand smoke metabolites that include 2'-Hydroxynicotine, 3-Pyridilacetic Acid, 3-Vinylpyridine, 4-(3-pyridil)-butanoic Acid, 4-(3-pyridyl)-3-butenoic acid, 4-(Methylamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-4-(3-pyridyl) butanal (NNA), 4-Oxo-4-(3-pyridyl)-N-methylbutanamide, 4-Oxo-4-(3-pyridyl)-butanamide, 4-Oxo-4-(3-pyridyl)-butanoic acid (OPBA), 4-hydroxy-4-(3-pyridyl)-butanoic acid (HPBA), 5'-Hydroxy-beta-Nicotyrine, 5'-Hydroxycotinine, 5-(3-pyridyl)-tetrahydrofuran-2-one, Cotinine, Cotinine Methonium Ion, Cotinine N-glucoronide, Cotinine N-oxide, N'- Hydroxymethyl- norcotinine, Nicotine Isomethonium Ion, Nicotine N'oxide, Nicotine N-glucoronide, Nicotine-delta 1' (5') Iminium Ion, Norcotinine, Nornicotine, Trans-3'-Hydroxycotinine, Trans-3'-Hydroxycotinine O glucoronide, and iso-NNAC.
S81 | THSTPS | Thirdhand Smoke Specific Metabolites | DOI:10.5281/zenodo.5394629
Nicotine has known human metabolites that include (S)-Nicotine iminium ion, 2'-Hydroxynicotine, and Nornicotine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Cotinine has a half life of 15-20 hours, while nicotine has a half life of 1-3 hours
Plasma concentrations of nicotine appear to decline in a biphasic manner. The half-life of nicotine in the initial phase is reportedly about 2-3 minutes and the half-life in the terminal phase reportedly averages about 2 hours (range: 1-4 hours).
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Following removal of a transdermal system of nicotine, plasma nicotine concentrations decline with an apparent half-life averaging 3-6 hours, which exceeds that of nicotine given iv; the slow decline in plasma nicotine concentrations following removal of a transdermal system appears to result from continued absorption of residual drug in the skin.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Cotinine is the major metabolite /of nicotine/ and has a plasma half-life of about 10-40 hours.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
... Following the administration of 0.1 mg nicotine/kg (labeled in 2-(14)C-pyrrolidone) to rats ... radioactivity due to nicotine and cotinine was detected in substantial amounts in plasma samples. Nicotine disappearance was biexponential, with an elimination half life of 1.0 hour. Cotinine appeared as the major metabolite in plasma and had elimination half life of 5.2 hours.
PMID:3667778 Kyerematen GA et al; J Chromatogr 419: 191-203 (1987)
Nicotine is a stimulant drug that acts as an agonist at nicotinic acetylcholine receptors. These are ionotropic receptors composed up of five homomeric or heteromeric subunits. In the brain, nicotine binds to nicotinic acetylcholine receptors on dopaminergic neurons in the cortico-limbic pathways. This causes the channel to open and allow conductance of multiple cations including sodium, calcium, and potassium. This leads to depolarization, which activates voltage-gated calcium channels and allows more calcium to enter the axon terminal. Calcium stimulates vesicle trafficking towards the plasma membrane and the release of dopamine into the synapse. Dopamine binding to its receptors is responsible the euphoric and addictive properties of nicotine. Nicotine also binds to nicotinic acetylcholine receptors on the chromaffin cells in the adrenal medulla. Binding opens the ion channel allowing influx of sodium, causing depolarization of the cell, which activates voltage-gated calcium channels. Calcium triggers the release of epinephrine from intracellular vesicles into the bloodstream, which causes vasoconstriction, increased blood pressure, increased heart rate, and increased blood sugar.
Nicotine is a ganglionic (nicotinic) cholinergic-receptor agonist. The pharmacologic actions of nicotine are complex and include a variety of effects mediated by stereospecific binding to receptors in autonomic ganglia, the adrenal medulla, the neuromuscular junction, and the brain.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
The principal pharmacologic effect of small doses of nicotine is initial, transient stimulation of autonomic ganglia; large doses or prolonged neuronal receptor exposure to nicotine results in subsequent persistent depression of receptor activity. Although nicotine has similar dose-related effects at the myoneural (neuromuscular) junction, rapidly developing skeletal muscle paralysis obscures the stimulant phase. The muscle-relaxant properties of nicotine may be mediated through stimulation of Renshaw cells and pulmonary afferent nerves, which results in inhibition of skeletal muscle motor activity; such relaxant effects may contribute to the behavior-reinforcing effects of the drug. Small doses of nicotine directly stimulate sympathetic ganglia and facilitate neurotransmission; however, large doses produce initial ganglionic stimulation, which is quickly followed by inhibition of neurotransmission.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Fruit Nikorono
Dosage Form : CHEWING
Dosage Strength : 2 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : NiQuitin Citrus
Dosage Form : COMPRESSED LOZENGES
Dosage Strength : 1.5 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : NiQuitin Mint
Dosage Form : CHEWING
Dosage Strength : 2 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Nicorette
Dosage Form : CHEWING
Dosage Strength : 2 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Nicorette Microtab
Dosage Form : SUBLINGUAL TABLET
Dosage Strength : 2 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Nicorette Novum
Dosage Form : TRANSDERMAL PATCH
Dosage Strength : 25 MG / 16 HOURS
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Nicorette Novum
Dosage Form : TRANSDERMAL PATCH
Dosage Strength : 10 MG / 16 HOURS
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Nicorette Peppermint
Dosage Form : OROMUCOSAL SOLUTION
Dosage Strength : 1 MG / SPRAY
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Regulatory Info :
Registration Country : Sweden
Brand Name : Nicotine Mint Apofri
Dosage Form : CHEWING
Dosage Strength : 2 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Sweden
Brand Name : NiQuitin Clear
Dosage Form : TRANSDERMAL PATCH
Dosage Strength : 21 MG / 24 H
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Company :
Nicotine
Drug Cost (USD) : 18,560,286
Year : 2022
Prescribers : 16670
Prescriptions : 32296

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 7,304,704
Year : 2022
Prescribers : 2913
Prescriptions : 10120

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 17,840,443
Year : 2021
Prescribers : 16635
Prescriptions : 32838

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 6,859,425
Year : 2021
Prescribers : 2589
Prescriptions : 9401

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 6,204,608
Year : 2020
Prescribers : 2340
Prescriptions : 9205

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 15,638,110
Year : 2020
Prescribers : 14306
Prescriptions : 30551

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 15,794,320
Year : 2019
Prescribers : 16593
Prescriptions : 32914

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 6,223,188
Year : 2019
Prescribers : 2813
Prescriptions : 10067

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 15,526,190
Year : 2018
Prescribers : 16243
Prescriptions : 32186

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Nicotine
Drug Cost (USD) : 6,141,482
Year : 2018
Prescribers : 2865
Prescriptions : 10479

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 2MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 4MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 4MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 2MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 4MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 2MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN :

Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 4MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Dosage Form : GUM, CHEWING;BUCCAL
Dosage Strength : EQ 2MG BASE
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

DIFGEN PHARMS
Dosage Form : FILM, EXTENDED RELEASE;TRANSDERM...
Dosage Strength : 21MG/24HR
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
DIFGEN PHARMS
Dosage Form : FILM, EXTENDED RELEASE;TRANSDERM...
Dosage Strength : 7MG/24HR
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : OTC

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
15 Nov 2024
Reply
12 Sep 2024
Reply
12 Sep 2022
Reply
10 Sep 2021
Reply
09 Jun 2021
Reply
13 May 2019
Reply
02 Apr 2019
Reply
22 Sep 2018
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2028-04-30
US Patent Number : 8323683
Drug Substance Claim :
Drug Product Claim :
Application Number : 18612
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2028-04-30

Patent Expiration Date : 2029-06-14
US Patent Number : 8501164
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 22360
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-06-14

Patent Expiration Date : 2028-04-30
US Patent Number : 8323683
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 20066
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2028-04-30

Patent Expiration Date : 2029-06-14
US Patent Number : 8501164
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 22360
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-06-14

Patent Expiration Date : 2029-04-30
US Patent Number : 8940772
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 22360
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-04-30

Patent Expiration Date : 2029-04-30
US Patent Number : 8940772
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 22360
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-04-30

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patent Expiration Date : 2014-09-16
Date Granted : 2005-08-23
Brand Name : NICORETTE INVISIPATCH
Patent Number : 2172402
Filing Date : 1994-09-16
Strength per Unit : 25 mg / 6 hour
Dosage Form : Transdermal patch
Human Or VET : Human
Route of Administration : Topically allied patch
Patent Expiration Date : 2014-09-16
Date Granted : 2005-08-23

Patent Expiration Date : 2011-11-15
Date Granted : 1994-11-15
Brand Name : PROSTEP TRANSDERMAL SYSTEM
Patent Number : 1333051
Filing Date : 1988-04-29
Strength per Unit : 11mg
Dosage Form : Patch
Human Or VET : Human
Route of Administration : Transdermal
Patent Expiration Date : 2011-11-15
Date Granted : 1994-11-15

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2011-11-15
Date Granted : 1994-11-15
Brand Name : PROSTEP TRANSDERMAL SYSTEM
Patent Number : 1333051
Filing Date : 1988-04-29
Strength per Unit : 22mg
Dosage Form : Patch
Human Or VET : Human
Route of Administration : Transdermal
Patent Expiration Date : 2011-11-15
Date Granted : 1994-11-15

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2006-05-23
Date Granted :
Brand Name :
Patent Number : 1254478
Filing Date :
Strength per Unit :
Dosage Form :
Human Or VET :
Route of Administration :
Patent Expiration Date : 2006-05-23
Date Granted :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
65
PharmaCompass offers a list of Nicotine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nicotine manufacturer or Nicotine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nicotine manufacturer or Nicotine supplier.
PharmaCompass also assists you with knowing the Nicotine API Price utilized in the formulation of products. Nicotine API Price is not always fixed or binding as the Nicotine Price is obtained through a variety of data sources. The Nicotine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nicotine D-Bitartrate Dihydrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nicotine D-Bitartrate Dihydrate, including repackagers and relabelers. The FDA regulates Nicotine D-Bitartrate Dihydrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nicotine D-Bitartrate Dihydrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nicotine D-Bitartrate Dihydrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nicotine D-Bitartrate Dihydrate supplier is an individual or a company that provides Nicotine D-Bitartrate Dihydrate active pharmaceutical ingredient (API) or Nicotine D-Bitartrate Dihydrate finished formulations upon request. The Nicotine D-Bitartrate Dihydrate suppliers may include Nicotine D-Bitartrate Dihydrate API manufacturers, exporters, distributors and traders.
click here to find a list of Nicotine D-Bitartrate Dihydrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nicotine D-Bitartrate Dihydrate DMF (Drug Master File) is a document detailing the whole manufacturing process of Nicotine D-Bitartrate Dihydrate active pharmaceutical ingredient (API) in detail. Different forms of Nicotine D-Bitartrate Dihydrate DMFs exist exist since differing nations have different regulations, such as Nicotine D-Bitartrate Dihydrate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nicotine D-Bitartrate Dihydrate DMF submitted to regulatory agencies in the US is known as a USDMF. Nicotine D-Bitartrate Dihydrate USDMF includes data on Nicotine D-Bitartrate Dihydrate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nicotine D-Bitartrate Dihydrate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nicotine D-Bitartrate Dihydrate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nicotine D-Bitartrate Dihydrate Drug Master File in Japan (Nicotine D-Bitartrate Dihydrate JDMF) empowers Nicotine D-Bitartrate Dihydrate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nicotine D-Bitartrate Dihydrate JDMF during the approval evaluation for pharmaceutical products. At the time of Nicotine D-Bitartrate Dihydrate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nicotine D-Bitartrate Dihydrate suppliers with JDMF on PharmaCompass.
A Nicotine D-Bitartrate Dihydrate CEP of the European Pharmacopoeia monograph is often referred to as a Nicotine D-Bitartrate Dihydrate Certificate of Suitability (COS). The purpose of a Nicotine D-Bitartrate Dihydrate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Nicotine D-Bitartrate Dihydrate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Nicotine D-Bitartrate Dihydrate to their clients by showing that a Nicotine D-Bitartrate Dihydrate CEP has been issued for it. The manufacturer submits a Nicotine D-Bitartrate Dihydrate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Nicotine D-Bitartrate Dihydrate CEP holder for the record. Additionally, the data presented in the Nicotine D-Bitartrate Dihydrate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Nicotine D-Bitartrate Dihydrate DMF.
A Nicotine D-Bitartrate Dihydrate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Nicotine D-Bitartrate Dihydrate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Nicotine D-Bitartrate Dihydrate suppliers with CEP (COS) on PharmaCompass.
A Nicotine D-Bitartrate Dihydrate written confirmation (Nicotine D-Bitartrate Dihydrate WC) is an official document issued by a regulatory agency to a Nicotine D-Bitartrate Dihydrate manufacturer, verifying that the manufacturing facility of a Nicotine D-Bitartrate Dihydrate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Nicotine D-Bitartrate Dihydrate APIs or Nicotine D-Bitartrate Dihydrate finished pharmaceutical products to another nation, regulatory agencies frequently require a Nicotine D-Bitartrate Dihydrate WC (written confirmation) as part of the regulatory process.
click here to find a list of Nicotine D-Bitartrate Dihydrate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nicotine D-Bitartrate Dihydrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nicotine D-Bitartrate Dihydrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nicotine D-Bitartrate Dihydrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nicotine D-Bitartrate Dihydrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nicotine D-Bitartrate Dihydrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nicotine D-Bitartrate Dihydrate suppliers with NDC on PharmaCompass.
Nicotine D-Bitartrate Dihydrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nicotine D-Bitartrate Dihydrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nicotine D-Bitartrate Dihydrate GMP manufacturer or Nicotine D-Bitartrate Dihydrate GMP API supplier for your needs.
A Nicotine D-Bitartrate Dihydrate CoA (Certificate of Analysis) is a formal document that attests to Nicotine D-Bitartrate Dihydrate's compliance with Nicotine D-Bitartrate Dihydrate specifications and serves as a tool for batch-level quality control.
Nicotine D-Bitartrate Dihydrate CoA mostly includes findings from lab analyses of a specific batch. For each Nicotine D-Bitartrate Dihydrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nicotine D-Bitartrate Dihydrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Nicotine D-Bitartrate Dihydrate EP), Nicotine D-Bitartrate Dihydrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nicotine D-Bitartrate Dihydrate USP).
