

Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
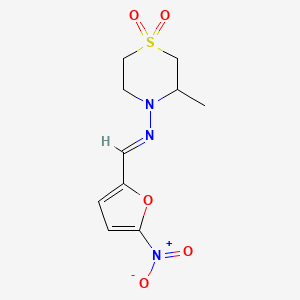

1. Bayer 2502
2. Lampit
1. Lampit
2. Bayer 2502
3. 23256-30-6
4. Nifurtimoxum
5. Bay 2502
6. Bayer-2502
7. Chebi:7566
8. Bay-a2502
9. Bay-2502
10. Dndi1613515
11. 4-thiomorpholinamine, 3-methyl-n-((5-nitro-2-furanyl)methylene)-, 1,1-dioxide
12. M84i3k7c2o
13. 4-((5-nitrofurfurylidene)amino)-3-methylthiomorpholine 1,1-dioxide
14. Thiomorpholine, 3-methyl-4-((5-nitrofurfurylidene)amino)-, 1,1-dioxide
15. 4-thiomorpholinamine, 3-methyl-n-[(5-nitro-2-furanyl)methylene]-, 1,1-dioxide
16. Nifurtimox [inn:ban]
17. Nifurtimoxum [inn-latin]
18. Ccris 2201
19. (+/-)-nifurtimox
20. X4kcv4zi9m
21. Sr-01000838852
22. Einecs 245-531-0
23. 1g5dd3p35c
24. Unii-m84i3k7c2o
25. 4-[(5-nitrofurfurylidene)amino]-3-methylthiomorpholine 1,1-dioxide
26. Bay A2502
27. (+)-nifurtimox
28. (-)-nifurtimox
29. Lampit (tn)
30. Nifurtimox, (+)-
31. Nifurtimox, (-)-
32. Nifurtimox [mi]
33. Nifurtimox [inn]
34. 4-((5-nitrofurfurylidene)amino)-3-methylthiomorpholine-1,1-dioxide
35. Nifurtimox (usan/inn)
36. Prestwick2_001024
37. Prestwick3_001024
38. Unii-x4kcv4zi9m
39. Nifurtimox [usan]
40. 1-((5-nitrofurfurylidene)amino)-2-methyltetrahydro-1,4-thiazine-4,4-dioxide
41. 3-methyl-n-[(5-nitro-2-furanyl)methylene]-4-thiomorpholinamine 1,1-dioxide
42. Nifurtimox [mart.]
43. Nifurtimox [who-dd]
44. Nifurtimox [who-ip]
45. Tetrahydro-3-methyl-4-((5-nitrofurfurylidene)amino)-2h-1,4-thiazine 1,1-dioxide
46. Unii-1g5dd3p35c
47. Bspbio_001207
48. Bpbio1_001329
49. Chembl290960
50. Schembl1650162
51. Nifurtimox [orange Book]
52. Nifurtimox, >=98% (hplc)
53. Chebi:91472
54. Bay2502
55. Kuc114565n
56. 39072-15-6
57. 39072-16-7
58. Ex-a4624
59. Nifurtimoxum [who-ip Latin]
60. Bdbm50259708
61. S6459
62. Cs-w020813
63. Db11820
64. Hy-w040073
65. Ksc-427-39-1
66. C08002
67. D00833
68. A912676
69. J-015055
70. Sr-01000838852-2
71. Sr-01000838852-3
72. Brd-a84020532-001-03-5
73. Brd-a84020532-001-04-3
74. (e)-3-methyl-4-(((5-nitrofuran-2-yl)methylene)amino)thiomorpholine 1,1-dioxide
75. 4-thiomorpholinamine, 3-methyl-n-((5-nitro-2-furanyl)methylene)-, 1,1-dioxide, (+)-
76. 4-thiomorpholinamine, 3-methyl-n-((5-nitro-2-furanyl)methylene)-, 1,1-dioxide, (-)-
77. N-(3-methyl-1,1-dioxo-1,4-thiazinan-4-yl)-1-(5-nitro-2-furyl)methanimine
78. Tetrahydro-3-methyl-4-((5-nitrofurfurylidene)amino)-4h-1,4-thiazine 1,1-dioxide
79. (rs)-3-methyl-n-((1e)-(5-nitro-2-furyl)methylene)thiomorpholin-4-amine 1,1-dioxide
| Molecular Weight | 287.29 g/mol |
|---|---|
| Molecular Formula | C10H13N3O5S |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 287.05759170 g/mol |
| Monoisotopic Mass | 287.05759170 g/mol |
| Topological Polar Surface Area | 117 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 467 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Nifurtimox is indicated in pediatric patients under 18 weighing at least 2.5 kg. Continued approval of this drug for this indication is dependent upon confirmatory clinical trial results.
Nifurtimox exerts trypanosomal activity against Trypanosoma cruzi, treating Chagas disease. One study reports that nifurtimox and other benzofuran derivatives reduce parasite dehydrogenase activity. Results of a recent phase III clinical trial have shown that a significant number of pediatric patients with acute or chronic Chagas disease treated with nifurtimox were immunoglobulin G (IgG) antibody negative and demonstrated at least a 20% decrease in optical density on two IgG antibody tests for T. cruzi antigens.
Trypanocidal Agents
Agents destructive to the protozoal organisms belonging to the suborder TRYPANOSOMATINA. (See all compounds classified as Trypanocidal Agents.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01C - Agents against leishmaniasis and trypanosomiasis
P01CC - Nitrofuran derivatives
P01CC01 - Nifurtimox
Absorption
The average AUC of nifurtimox is estimated between 1676-2670 gh/L. One pharmacokinetic study of healthy volunteers revealed an AUC of 5430 ngml-1h. Cmax ranges between 425-568 g/L (2650%) after a single dose of 20 mg with food in adults. Tmax is 4 hours, ranging from 2 to 8 hours post-dose in the fed state. In a pharmacokinetic study of healthy volunteers, serum concentration was low, likely due to the first-pass effect.
Route of Elimination
In the fed state, 44% of the dose was mainly recovered in the urine as metabolites. Fecal and biliary excretion of nifurtimox have not been studied.
Volume of Distribution
Nifurtimox crosses the blood-brain barrier and the placenta.
Clearance
One pharmacokinetic study of nifurtimox revealed a clearance of 193.4 lh-1. In patients without renal failure; clearance was 99.7 lh-1.
Nifurtimox is largely metabolized via nitroreductase enzymes. Two major inactive metabolites have been identified: M-4 and M-6. The M-4 metabolite is a cysteine conjugate of nifurtimox, while M-6 is likely formed by hydrolytic cleavage of the hydrazone moiety of nifurtimox. Other minor metabolites have also been identified in human plasma.
The elimination half-life of nifurtimox ranges from 2.43.6 hours. A pharmacokinetic study of healthy volunteers and patients with renal failure revealed respective mean half-lives of 2.95 h and 3.95 h.
The mechanism of action of nifurtimox has not been fully elucidated, however, is believed to occur by the activation of nitroreductase enzymes that produce reactive metabolites with a series of deleterious effects on Trypanosoma cruzi, the parasite causing Chagas disease. The antiprotozoal actions of nifurtimox occur both intracellularly and extracellularly. Inhibition of parasite dehydrogenase activity is another purported mode of action of nifurtimox that warrants further research.
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
