

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
FDF
0
Australia

0
Listed Dossiers
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
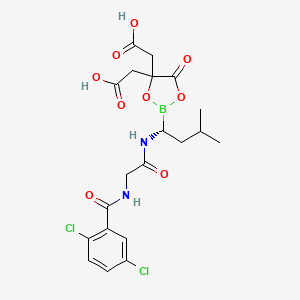

1. 1239908-20-3
2. Ixazomib Citrate (ester)
3. Ixazomib Citrate [usan]
4. 46cwk97z3k
5. 2-[4-(carboxymethyl)-2-[(1r)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methylbutyl]-5-oxo-1,3,2-dioxaborolan-4-yl]acetic Acid
6. Unii-46cwk97z3k
7. 1,3,2-dioxaborolane-4,4-diacetic Acid, 2-((1r)-1-((2-((2,5-dichlorobenzoyl)amino)acetyl)amino)-3-methylbutyl)-5-oxo-
8. 1,3,2-dioxaborolane-4,4-diacetic Acid, 2-[(1r)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methylbutyl]-5-oxo-
9. 2,2'-(2-((1r)-1-((n-(2,5-dichlorobenzoyl)glycyl)amino)-3-methylbutyl)-5-oxo-1,3,2-dioxaborolane-4,4-diyl)diacetic Acid
10. 2,2'-{2-[(1r)-1-{[n-(2,5-dichlorobenzoyl)glycyl]amino}-3-methylbutyl]-5-oxo-1,3,2-dioxaborolane-4,4-diyl}diacetic Acid
11. Ninlaro (tn)
12. Ixazomib Citrate [mi]
13. Ixazomib Citrate (jan/usan)
14. Ixazomib Citrate [jan]
15. Schembl4427479
16. Chembl3545432
17. Chebi:90939
18. Dtxsid60924652
19. Ixazomib Citrate [who-dd]
20. Bcp16600
21. Ex-a3660
22. Nsc767907
23. S4432
24. Akos030632768
25. Ixazomib Citrate [orange Book]
26. Zinc200299610
27. Cs-1720
28. Nsc-767907
29. Ncgc00522453-01
30. Ncgc00522453-02
31. 2,2'-{2-[(1r)-1-({[(2,5-dichlorobenzoyl)amino]acetyl}amino)-3-methylbutyl]-5-oxo-1,3,2-dioxaborolane-4,4-diyl}diacetic Acid
32. Ac-35344
33. As-75031
34. Hy-10452
35. C71946
36. D10131
37. Mln9708; Mln-9708; Mln 9708;ixazomib-prodrug
38. Q27162929
39. (r)-2,2'-(2-(1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutyl)-5-oxo-1,3,2-dioxaborolane-4,4-diyl)diacetic Acid
40. 2,2'-{2-[(1r)-1-({[(2,5-dichlorobenzoy)amino]acetyl}amino)-3-methylbutyl]-5-oxo-1,3,2-dioxaborolane-4,4-diyl}diacetic Acid
41. 2-[(1r)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methylbutyl]-5-oxo-1,3,2-dioxaborolane-4,4-diacetic Acid
42. 2-[4-(carboxymethyl)-2-[(1r)-1-{2-[(2,5-dichlorophenyl)formamido]acetamido}-3-methylbutyl]-5-oxo-1,3,2-dioxaborolan-4-yl]acetic Acid
1. Ixazomib
| Molecular Weight | 517.1 g/mol |
|---|---|
| Molecular Formula | C20H23BCl2N2O9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 11 |
| Exact Mass | 516.0873659 g/mol |
| Monoisotopic Mass | 516.0873659 g/mol |
| Topological Polar Surface Area | 168 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 797 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of systemic light chain amyloidosis

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
