
Synopsis
Synopsis
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
Listed Suppliers
0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Endogenous Nitrate Vasodilator
2. Endothelium-derived Nitric Oxide
3. Mononitrogen Monoxide
4. Monoxide, Mononitrogen
5. Monoxide, Nitrogen
6. Nitrate Vasodilator, Endogenous
7. Nitric Oxide, Endothelium Derived
8. Nitric Oxide, Endothelium-derived
9. Nitrogen Monoxide
10. Oxide, Nitric
11. Vasodilator, Endogenous Nitrate
1. Nitrogen Monoxide
2. Mononitrogen Monoxide
3. 10102-43-9
4. Inomax
5. Nitrosyl Radical
6. Nitrogen Oxide (no)
7. Amidogen, Oxo-
8. Nitrosyl
9. Nitrogen Monooxide
10. Nitric Oxide Trimer
11. Inovent
12. Edrf
13. Chebi:16480
14. Endothelium-derived Relaxing Factor
15. Ohm-11771
16. Monoxyde D'azote
17. Oxoazanyl
18. Oxido Nitrico
19. Oxyde Azotique
20. Stickstoffmonoxid
21. Nitrogen(ii) Oxide
22. Monoxido De Nitrogeno
23. Stickstoff(ii)-oxid
24. Inomax (tn)
25. Oxido De Nitrogeno(ii)
26. Nitrosoradical
27. [no]
28. Oxidonitrogen(.)
29. Ohm 11771
30. Genosyl
31. Nitric Oxide, 98.5%
32. Nitric Oxide [mi]
33. Nitric Oxide (jan/usan)
34. Nitric Oxide [jan]
35. Nitric Oxide [hsdb]
36. Nitric Oxide [vandf]
37. Nitric Oxide [mart.]
38. Nitric Oxide [who-dd]
39. Chembl1200689
40. Dtxsid1020938
41. Nitric Oxide [ema Epar]
42. (.)no
43. No(.)
44. Nitric Oxide [orange Book]
45. Nitric Oxide [ep Monograph]
46. (no)(.)
47. Db00435
48. C00533
49. D00074
| Molecular Weight | 30.006 g/mol |
|---|---|
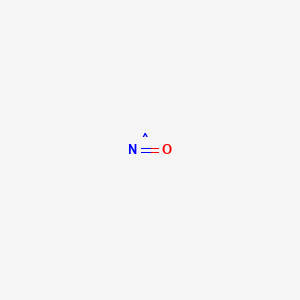
| Molecular Formula | NO |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 29.997988624 g/mol |
| Monoisotopic Mass | 29.997988624 g/mol |
| Topological Polar Surface Area | 18.1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Inomax |
| PubMed Health | Nitric Oxide (By breathing) |
| Drug Label | INOmax (nitric oxide gas) is a drug administered by inhalation. Nitric oxide, the active substance in INOmax, is a pulmonary vasodilator. INOmax is a gaseous blend of nitric oxide and nitrogen (0.08% and 99.92%, respectively for 800 ppm; 0.01% and 99... |
| Active Ingredient | Nitric oxide |
| Dosage Form | Gas |
| Route | Inhalation |
| Strength | 800ppm; 100ppm |
| Market Status | Prescription |
| Company | Ino |
| 2 of 2 | |
|---|---|
| Drug Name | Inomax |
| PubMed Health | Nitric Oxide (By breathing) |
| Drug Label | INOmax (nitric oxide gas) is a drug administered by inhalation. Nitric oxide, the active substance in INOmax, is a pulmonary vasodilator. INOmax is a gaseous blend of nitric oxide and nitrogen (0.08% and 99.92%, respectively for 800 ppm; 0.01% and 99... |
| Active Ingredient | Nitric oxide |
| Dosage Form | Gas |
| Route | Inhalation |
| Strength | 800ppm; 100ppm |
| Market Status | Prescription |
| Company | Ino |
Bronchodilator Agents; Free Radical Scavengers; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Although its use still is considered experimental, nitric oxide has been used successfully in some patients with persistent fetal circulation, pulmonary hypertension secondary to cardiac dysfunction or surgery, or with the adult respiratory distress syndrome.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 356
For the treatment of term and near-term (>34 weeks) neonates with hypoxic respiratory failure
INOmax, in conjunction with ventilatory support and other appropriate active substances, is indicated:
- for the treatment of newborn infants 34 weeks gestation with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension, in order to improve oxygenation and to reduce the need for extracorporeal membrane oxygenation;
- as part of the treatment of peri- and post-operative pulmonary hypertension in adults and newborn infants, infants and toddlers, children and adolescents, ages 0-17 years in conjunction to heart surgery, in order to selectively decrease pulmonary arterial pressure and improve right ventricular function and oxygenation.
Persistent pulmonary hypertension of the newborn (PPHN) occurs as a primary developmental defect or as a condition secondary to other diseases such as meconium aspiration syndrome (MAS), pneumonia, sepsis, hyaline membrane disease, congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia. In these states, pulmonary vascular resistance (PVR) is high, which results in hypoxemia secondary to right-to-left shunting of blood through the patent ductus arteriosus and foramen ovale. In neonates with PPHN, Nitric oxide improves oxygenation (as indicated by significant increases in PaO2). Nitric oxide appears to increase the partial pressure of arterial oxygen (PaO2) by dilating pulmonary vessels in better entilated areas of the lung, redistributing pulmonary blood flow away from lung regions with low ventilation/perfusion (V/Q) ratios toward regions with normal ratios.
Endothelium-Dependent Relaxing Factors
Paracrine substances produced by the VASCULAR ENDOTHELIUM with VASCULAR SMOOTH MUSCLE relaxation (VASODILATION) activities. Several factors have been identified, including NITRIC OXIDE and PROSTACYCLIN. (See all compounds classified as Endothelium-Dependent Relaxing Factors.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Neurotransmitter Agents
Substances used for their pharmacological actions on any aspect of neurotransmitter systems. Neurotransmitter agents include agonists, antagonists, degradation inhibitors, uptake inhibitors, depleters, precursors, and modulators of receptor function. (See all compounds classified as Neurotransmitter Agents.)
Free Radical Scavengers
Substances that eliminate free radicals. Among other effects, they protect PANCREATIC ISLETS against damage by CYTOKINES and prevent myocardial and pulmonary REPERFUSION INJURY. (See all compounds classified as Free Radical Scavengers.)
Gasotransmitters
Endogenously produced lipid-soluble gaseous molecules which function as neurotransmitters and signal mediators targeting ION CHANNELS and transporters. (See all compounds classified as Gasotransmitters.)
R07AX
R - Respiratory system
R07 - Other respiratory system products
R07A - Other respiratory system products
R07AX - Other respiratory system products
R07AX01 - Nitric oxide
Absorption
Nitric oxide is absorbed systemically after inhalation.
Route of Elimination
Nitrate has been identified as the predominant nitric oxide metabolite excreted in the urine, accounting for >70% of the nitric oxide dose inhaled.
ABSORPTION IS BY WAY OF LUNG.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 189
ON INHALATION, CONSIDERABLE PORTION ... IS ABSORBED IN UPPER RESP TRACT.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 4099
Most of the inhaled nitric oxide is eventually eliminated from the body as nitrate.
Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 512
... it is efficiently (>80%) absorbed via inhalation ...
Sullivan, J.B. Jr., G.R. Krieger (eds.). Hazardous Materials Toxicology-Clinical Principles of Environmental Health. Baltimore, MD: Williams and Wilkins, 1992., p. 965
When seven volunteers inhaled 0.33, 0.5, 1, or 5 ppm, 85% to 93% of the inspired nitric oxide was retained.
American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 1091
via pulmonary capillary bed
The biotransformation of nitric oxide (NO) and its intermed metabolites, nitrite and nitrate ions, was reviewed: absorption and conversion of NO in blood, metabolism and excretion of inhaled NO, and conversion of nitrite and nitrate in the digestive system. ... The major proportion of inhaled NO reaches the deeper portion of the lung and reacts with hemoglobin in erythrocytes to form nitrosylhemoglobin which is converted immediately to nitrite and nitrate. The nitrate and nitrite are then transferred to the serum, and the greater part of the nitrate is excreted into the urine through the kidney. ... Part of the nitrate in the blood is secreted into the oral cavity through saliva and is converted to nitrite by oral bacteria, part of the nitrite that reaches the stomach is converted to nitrogen gas with the proteins of the diet and disappears, the intestinal nitrate transferred from the blood and stomach is converted to ammonia or unknown compounds through nitrite by the intestinal bacteria, the thus produced ammonia is absorbed through the intestinal wall into the body, and this ammonia is metabolized to urea through the urea cycle and excreted into the urine.
PMID:3665863 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1474568 Yoshida K, Kasama K; Environ Health Perspect 73: 201-205 (1987)
2–6 seconds
Nitric oxide is a compound produced by many cells of the body. It relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3',5'-monophosphate, which then leads to vasodilation. When inhaled, nitric oxide produces pulmonary vasodilation.
The effect of nitric oxide (NO) on blood hemoglobin was studied in vitro. Previously deoxygenated human blood was equilibrated with a continuous flow of 1,000 ppm NO, 5.6% carbon dioxide in nitrogen for 3 hours, and flushed for 30 min with 5.6% carbon dioxide in nitrogen. Nitrosylhemoglobin containing blood was equilibrated for various lengths of time with 21% oxygen and 5.6% carbon dioxide in nitrogen or 100% carbon monoxide for 1 hr. Methemoglobin was measured with an anaerobic method. Carboxyhemoglobin was measured. Anaerobic nitrosylhemoglobin blood/buffer solution was equilibratd with carbon monoxide and nitrogen. Acid/base blood changes in nitrosylhemoglobin and methemoglbin formation were determined after 1 hr of nitrogen equilibration, after 3 hr of NO equilibration, and after air exposure. The amount of methemoglobin formed was monitored by spectrophotometric changes of the anaerobic nitrosylhemoglobin solution after being exposed to air. Nitrosylhemoglobin exposure to oxygen incr the methemoglobin formation, with 59% and 78% of the total hemoglobin oxidized in the first 15 min and after 120 min of oxygen exposure, respectively. No significant methemoglobin was formed with nitrosylhemoglobin exposure to carbon dioxide. A total of 42% total hemoglobin in the nitrosylhemoglobin in the nitrosylhemoglobin blood/buffer solution was converted to methemoglobin after 30 min of air exposure and to 55% after 60 min of air exposure. NO exposure significantly decr the pH and base excess after 3 hr of exposure to NO. There were no significant changes after air exposure. ... /It was concluded/ that only under strict anaerobic conditions can NO combine with hemoglobin as nitrosylhemoglobin without any methemoglobin. NO is not a hemoglobin oxidant, it needs the presence of oxygen.
PMID:4017990 Chiodi H, Mohler JG; Environ Res 37 (2): 355-63 (1985)
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
29
PharmaCompass offers a list of Nitric Oxide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nitric Oxide manufacturer or Nitric Oxide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nitric Oxide manufacturer or Nitric Oxide supplier.
PharmaCompass also assists you with knowing the Nitric Oxide API Price utilized in the formulation of products. Nitric Oxide API Price is not always fixed or binding as the Nitric Oxide Price is obtained through a variety of data sources. The Nitric Oxide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nitric Oxide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nitric Oxide, including repackagers and relabelers. The FDA regulates Nitric Oxide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nitric Oxide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Nitric Oxide supplier is an individual or a company that provides Nitric Oxide active pharmaceutical ingredient (API) or Nitric Oxide finished formulations upon request. The Nitric Oxide suppliers may include Nitric Oxide API manufacturers, exporters, distributors and traders.
click here to find a list of Nitric Oxide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nitric Oxide DMF (Drug Master File) is a document detailing the whole manufacturing process of Nitric Oxide active pharmaceutical ingredient (API) in detail. Different forms of Nitric Oxide DMFs exist exist since differing nations have different regulations, such as Nitric Oxide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nitric Oxide DMF submitted to regulatory agencies in the US is known as a USDMF. Nitric Oxide USDMF includes data on Nitric Oxide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nitric Oxide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nitric Oxide suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Nitric Oxide Drug Master File in Korea (Nitric Oxide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Nitric Oxide. The MFDS reviews the Nitric Oxide KDMF as part of the drug registration process and uses the information provided in the Nitric Oxide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Nitric Oxide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Nitric Oxide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Nitric Oxide suppliers with KDMF on PharmaCompass.
A Nitric Oxide CEP of the European Pharmacopoeia monograph is often referred to as a Nitric Oxide Certificate of Suitability (COS). The purpose of a Nitric Oxide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Nitric Oxide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Nitric Oxide to their clients by showing that a Nitric Oxide CEP has been issued for it. The manufacturer submits a Nitric Oxide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Nitric Oxide CEP holder for the record. Additionally, the data presented in the Nitric Oxide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Nitric Oxide DMF.
A Nitric Oxide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Nitric Oxide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Nitric Oxide suppliers with CEP (COS) on PharmaCompass.
Nitric Oxide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nitric Oxide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nitric Oxide GMP manufacturer or Nitric Oxide GMP API supplier for your needs.
A Nitric Oxide CoA (Certificate of Analysis) is a formal document that attests to Nitric Oxide's compliance with Nitric Oxide specifications and serves as a tool for batch-level quality control.
Nitric Oxide CoA mostly includes findings from lab analyses of a specific batch. For each Nitric Oxide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nitric Oxide may be tested according to a variety of international standards, such as European Pharmacopoeia (Nitric Oxide EP), Nitric Oxide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nitric Oxide USP).
