
Synopsis
Synopsis
0
EU WC
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Anginine
2. Dynamite
3. Gilustenon
4. Glyceryl Trinitrate
5. Nitrangin
6. Nitro Bid
7. Nitro Dur
8. Nitro-bid
9. Nitro-dur
10. Nitrobid
11. Nitrocard
12. Nitroderm
13. Nitroderm Tts
14. Nitrodur
15. Nitroglyn
16. Nitrol
17. Nitrolan
18. Nitrong
19. Nitrospan
20. Nitrostat
21. Perlinganit
22. Susadrin
23. Sustac
24. Sustak
25. Sustonit
26. Transderm Nitro
27. Tridil
28. Trinitrate, Glyceryl
29. Trinitrin
30. Trinitrolong
1. Glyceryl Trinitrate
2. Trinitroglycerin
3. Nitrostat
4. 55-63-0
5. Nitroglycerine
6. Nitroglycerol
7. Glycerol Trinitrate
8. Nitro-dur
9. Minitran
10. Trinitroglycerol
11. Trinitrin
12. Tridil
13. Transderm-nitro
14. Nitrolingual
15. Nitroderm
16. Nitroglyn
17. Nitronet
18. Nitrospan
19. Nitora
20. Transderm Nitro
21. Nitro-bid
22. Epinitril
23. Lenitral
24. Natispray
25. Nitrocine
26. Nitrolan
27. Perlinganit
28. Susadrin
29. Sustonit
30. Nitrong
31. Niong
32. Nitromist
33. Nitroderm Tts
34. Propane-1,2,3-triyl Trinitrate
35. Angiolingual
36. Cordipatch
37. Corditrine
38. Glycerin Trinitrate
39. Klavikordal
40. Myoglycerin
41. Nitroglicerina
42. Nitroletten
43. Nitroplast
44. Nitrorectal
45. Nitroretard
46. Nitrostabilin
47. Nysconitrine
48. Perglottal
49. Rectogesic
50. Temponitrin
51. Trinitrosan
52. Adesitrin
53. Angibid
54. Angorin
55. Cardamist
56. Chitamite
57. Deponit
58. Glonoin
59. Glytrin
60. Millisrol
61. Niglycon
62. Nitrodisc
63. Nitrogard
64. Nitroglin
65. Nitrolowe
66. Nitromel
67. Nitromex
68. Nitromint
69. Nitronal
70. Percutol
71. Suscard
72. Trinalgon
73. Triniplas
74. Trinitrol
75. Vasoglyn
76. Myocon
77. Niglin
78. Gilucor Nitro
79. Nitrocor
80. Soup
81. Blasting Oil
82. Blasting Gelatin
83. Glyceryl Nitrate
84. Nitro-lent
85. Nitrozell Retard
86. Transiderm-nitro
87. Coro-nitro
88. Nitro-span
89. Nitrine-tdc
90. Spirit Of Glonoin
91. Nitrolingual Spray
92. 1,2,3-propanetriol, Trinitrate
93. Nitrogliceryna
94. Glonoinum
95. Rectiv
96. Transderm-nitro Tts
97. Glyceroltrinitraat
98. Propanetriol Trinitrate
99. Nitro Iv
100. 1,2,3-propanetriyl Nitrate
101. Glycerol, Nitric Acid Triester
102. Gonitro
103. 1,3-dinitrooxypropan-2-yl Nitrate
104. Nitroglycerin, Spirits Of
105. Spirit Of Trinitroglycerin
106. Ng
107. Rcra Waste Number P081
108. Sk-106n
109. Solution Glyceryl Trinitrate
110. Spirit Of Glyceryl Trinitrate
111. Nk-843
112. Trinitrine
113. Glycerol(trinitrate De)
114. 1,2,3-propanetriol Trinitrate
115. Ntg
116. Imx-150
117. 1,2,3-propanetrioltrinitrate
118. Glycerol Nitric Acid Triester
119. Diluted Nitroglycerin
120. C01da02
121. Gilustenon
122. Trinitrolong
123. Sustak
124. Gtn
125. Nitrobid
126. 1,2,3-propanetriol, 1,2,3-trinitrate
127. G59m7s0ws3
128. Aldonitrin
129. Cardiodisco
130. Colenitral
131. Discotrine
132. Glycerylnitrat
133. Lentonitrina
134. Nitrobukal
135. Nitrocerin
136. Nitrocontin
137. Nitropatch
138. Nitropercuten
139. Nitroperlinit
140. Nitropront
141. Nitroprontan
142. Plastranit
143. Ratiopharm
144. Tng
145. Trinipatch
146. Angiplex
147. Angonist
148. Buccard
149. Cardabid
150. Cardinit
151. Dauxona
152. Diafusor
153. Dtxsid1021407
154. Glyceryl
155. Herwicard
156. Minitram
157. Mionitrat
158. Nitradisc
159. Nitroard
160. Nitrobaat
161. Nitroclyn
162. Nitrocot
163. Nitrodyl
164. Nitrolin
165. Nitropen
166. Nitroprol
167. Nitrorex
168. Nitrovis
169. Perganit
170. Polnitrin
171. Trinitron
172. Turicard
173. Vasolator
174. Vernies
175. Willong
176. Anglix
177. Chebi:28787
178. Herzer
179. Myovin
180. Nirmin
181. Nitrek
182. Sustac
183. Nitrol Ointment
184. Trinitrina Erba
185. Nitradisc Pad
186. Nitro-pflaster
187. Nitrobid Oint
188. Nitrong Retard
189. Niong Retard
190. Nitro Retard
191. Aquo-trimitrosan
192. Nitro Rorer
193. Nitromack Retard
194. Nitromint Retard
195. Nitronal Aqueous
196. Nitro-time
197. Nitrogard-sr
198. Mi-trates
199. Nitradisc Tts
200. Nitriderm Tts
201. Nitro-par
202. Top-nitro
203. Nitrocine 5
204. Nitrodyl Tts
205. Nitromint Aerosol
206. Nitrong-sr
207. Deponit 5
208. Deponit-5
209. Neos Nitro Opt
210. Nit-ret
211. Nitroglycerin-acc
212. Transderm-n Tts
213. Nitro Mack Retard
214. Nitro-m-bid
215. Nitro-mack Retard
216. Gepan Nitroglicerin
217. Nitro Dur Tts
218. Nitroderm Tts-5
219. Gtn-pohl
220. Nitroderm Tts Ext
221. Nitro-dur 5
222. Nitrocontin Continus
223. Deponit Tts 5
224. Nitro-gesanit Retard
225. Cellegesic
226. Deponit Tts 10
227. Nitro-dur 10
228. Nitrocap
229. Nitroquick
230. Nitroquik
231. Glycerintrinitrate
232. Percutol Oint.
233. Nitrong Parenteral
234. Nitrocapt.d.
235. Glycerine Trinitrate
236. Nitroglycerin (ng)
237. Corangin Nitrokapseln
238. Trinitroglicerina Fabra
239. Anogesic
240. Minitro
241. Nitriderm
242. Nitroject
243. Vascana
244. Buccal
245. Nitrogliceryna [polish]
246. Nitroglycerine [french]
247. Nitrol (pharmaceutical)
248. Tridil Sublin
249. Trinitrine Simple Laleuf
250. Nitroglicerina [italian]
251. Nitroglicerina [spanish]
252. Aquo-trinitrosan
253. Glycerintrinitrate [czech]
254. Glyceroltrinitraat [dutch]
255. Trinitrin Tablets
256. Nitrolingual Pumpspray
257. Nitromist (tn)
258. Minitran (tn)
259. Hsdb 30
260. Sdm No. 17
261. Nitroglycerin In Dextrose 5%
262. Nitro-bid (tn)
263. Nitro-dur (tn)
264. Ccris 4089
265. Nitroglycerin Ointment
266. Nitric Acid Triester Of Gylcerol
267. Transderm-nitro (tn)
268. Glycerol(trinitrate De) [french]
269. Glycerol (trinitrate De) [french]
270. Glycerol (trinitrate De)
271. Einecs 200-240-8
272. Un0143
273. Un1204
274. Un3064
275. Un3319
276. Nitroglycerin [usp:jan]
277. Rcra Waste No. P081
278. Brn 1802063
279. Unii-g59m7s0ws3
280. 2,3-dinitrooxypropyl Nitrate
281. Nitrofortin
282. Nitrolande
283. Nitrosigma
284. Reminitrol
285. Solinitrina
286. Spirits Of Nitroglycerin, (1 To 10%)
287. Corangil
288. Nitrolar
289. Nitromed
290. Sutonit
291. Myocor
292. Trans-nitro
293. Glonoine Oil
294. Nitro Mack
295. S.n.g.
296. 2,3-bis(nitrooxy)propyl Nitrate
297. Mqx-503
298. Nitro-prn
299. Med-2002
300. Nitroglycerin Tablets
301. Sk-866
302. Sk-878
303. Un0144
304. Nitroglycerin Injection
305. Spirits Of Nitroglycerin
306. Glonoinum [hpus]
307. Nitroglycerine, Spirit Of
308. 1,2,3-trinitrooxypropane
309. Nitroglycerin [mi]
310. Chembl730
311. Ec 200-240-8
312. Glyceryl Trinitrate Solution
313. Nitroglycerin (jp17/usp)
314. Nitroglycerin [hsdb]
315. 1,2,3-trinitroglycerin-d5
316. Schembl15421
317. Nitroglycerin [vandf]
318. 4-01-00-02762 (beilstein Handbook Reference)
319. Un 0143 (salt/mix)
320. Un 0144 (salt/mix)
321. Un 1204 (salt/mix)
322. Bidd:gt0142
323. Nitroglycerin, Liquid, Not Desensitized [forbidden]
324. Sdm No. 17 (salt/mix)
325. Sdm No. 27 (salt/mix)
326. 1,2,3-propanetriol Trinatrate
327. 1,2,3-tris-nitryloxy-propane
328. Gtpl7053
329. Trinitrate 1,2,3-propanetriol
330. Nitric Acid Triester Of Glycerol
331. Hms2094m15
332. Nitroglycerin [orange Book]
333. Nitroglycerin Tablets [jan]
334. Glyceryl Trinitrate [mart.]
335. Zinc8214625
336. Nitroglycerin [usp Impurity]
337. C0061
338. Glyceryl Trinitrate [who-dd]
339. Akos030254238
340. Diluted Nitroglycerin [usp-rs]
341. Nitroglycerin, Solution In Alcohol With > 5% But Not > 10% Nitroglycerin
342. Db00727
343. Nitroglycerin, Liquid, Not Desensitized
344. Diluted Nitroglycerin [usp Monograph]
345. Ft-0752897
346. C07455
347. D00515
348. Glyceryl Trinitrate Solution [ep Monograph]
349. Q162867
350. Sr-01000944510
351. Sr-01000944510-1
352. Nitroglycerin, Solution In Alcohol, With Not >1% Nitroglycerin
353. Nitroglycerin, Solution In Alcohol, With >1% But Not >10% Nitroglycerin
354. Nitroglycerin, Solution In Alcohol, With >1% But Not >5% Nitroglycerin
355. Nitroglycerin Mixture With >2% But Not >10% Nitroglycerin, By Mass, Desensitized
356. Nitroglycerin, Desensitized With Not <40% Non-volatile Water Insoluble Phlegmatizer, By Mass
357. Nitroglycerin 100 Microg/ml In Acetonitrile. Short Expiry Date Due To Chemical Nature Of Component(s)
358. Nitroglycerin Mixture With >2% But Not >10% Nitroglycerin, By Mass, Desensitized [un3319] [flammable Solid]
359. Nitroglycerin, Desensitized With Not <40% Non-volatile Water Insoluble Phlegmatizer, By Mass [un0143] [explosive 1.1d, Poison]
360. Nitroglycerin, Solution In Alcohol, With >1% But Not >10% Nitroglycerin [un0144] [explosive 1.1d]
361. Nitroglycerin, Solution In Alcohol, With >1% But Not >5% Nitroglycerin [un3064] [flammable Liquid]
362. Nitroglycerin, Solution In Alcohol, With Not >1% Nitroglycerin [un1204] [flammable Liquid]
363. Trinitroglycerin Solution, 1 % (w/w) In Propylene Glycol, Ampule Of 1 Ml, Certified Reference Material
364. Trinitroglycerin Solution, 1 % (w/w) In Propylene Glycol, Ampule Of 5 X 0.2 Ml, Certified Reference Material
365. Trinitroglycerin Solution, 1000 Mug/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 227.09 g/mol |
|---|---|
| Molecular Formula | C3H5N3O9 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 227.00257875 g/mol |
| Monoisotopic Mass | 227.00257875 g/mol |
| Topological Polar Surface Area | 165 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 219 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 14 | |
|---|---|
| Drug Name | Minitran |
| PubMed Health | Nitroglycerin |
| Drug Classes | Antianginal, Cardiovascular Agent, Colorectal Agent, Coronary Vasodilator, Nitrate |
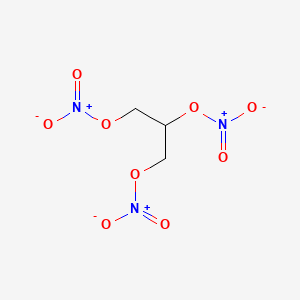
| Drug Label | Nitroglycerin is a 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula isand whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.The MINITRAN (nitroglycerin) Transdermal... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 0.1mg/hr; 0.6mg/hr; 0.4mg/hr; 0.2mg/hr |
| Market Status | Prescription |
| Company | Valeant Pharm North; Medicis |
| 2 of 14 | |
|---|---|
| Drug Name | Nitro-dur |
| PubMed Health | Nitroglycerin Patch (Absorbed through the skin) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.The NITRO-DUR (nitroglycerin) Transdermal... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 0.1mg/hr; 0.6mg/hr; 0.4mg/hr; 0.2mg/hr; 0.8mg/hr; 0.3mg/hr |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 3 of 14 | |
|---|---|
| Drug Name | Nitroglycerin |
| PubMed Health | Nitroglycerin |
| Drug Classes | Antianginal, Cardiovascular Agent, Colorectal Agent, Coronary Vasodilator, Nitrate |
| Drug Label | Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.The NITRO-DUR (nitroglycerin) Transdermal... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Ointment; Spray, metered; Film, extended release; Injectable |
| Route | Injection; Sublingual; transdermal; Transdermal |
| Strength | 0.1mg/hr; 0.6mg/hr; 0.4mg/hr; 0.2mg/hr; 0.4mg/spray; 5mg/ml; 2% |
| Market Status | Tentative Approval; Prescription |
| Company | Noven; Hercon Pharm; Fougera; Luitpold; Mylan Technologies; Perrigo Israel |
| 4 of 14 | |
|---|---|
| Drug Name | Nitrolingual pumpspray |
| PubMed Health | Nitroglycerin |
| Drug Classes | Antianginal, Cardiovascular Agent, Colorectal Agent, Coronary Vasodilator, Nitrate |
| Drug Label | Nitroglycerin, an organic nitrate, is a vasodilator which has effects on both arteries and veins. The chemical name for nitroglycerin is 1,2,3-propanetriol trinitrate (C3H5N3O9). The compound has a molecular weight of 227.09. The chemical structure i... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Spray, metered |
| Route | Sublingual |
| Strength | 0.4mg/spray |
| Market Status | Prescription |
| Company | Pohl Boskamp |
| 5 of 14 | |
|---|---|
| Drug Name | Nitromist |
| Drug Label | Nitroglycerin, an organic nitrate, is a vasodilator which has effects on both arteries and veins. The chemical name for nitroglycerin is 1,2,3-propanetriol trinitrate (C3H5N3O9). The compound has a molecular weight of 227.09. The chemical structure i... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Aerosol, metered |
| Route | Sublingual |
| Strength | 0.4mg/spray |
| Market Status | Prescription |
| Company | Mist Pharms |
| 6 of 14 | |
|---|---|
| Drug Name | Nitrostat |
| Drug Label | NITROSTAT is a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg , or 0.6 mg nitroglycerin; as well as lactose monohydrate, NF; glyceryl monostearate, NF; pregelatinized starch, NF; calcium stearate, NF powder; and si... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Tablet |
| Route | Sublingual |
| Strength | 0.4mg; 0.6mg; 0.3mg |
| Market Status | Prescription |
| Company | Pfizer Pharms |
| 7 of 14 | |
|---|---|
| Drug Name | Rectiv |
| Drug Label | Nitroglycerin is 1,2,3,-propanetriol trinitrate, an organic nitrate whose structural formula is as follows:CH2-ONO2|CH-ONO2|CH2-ONO2and whose molecular weight is 227.09. RECTIV (nitroglycerin) Ointment 0.4% contains 0.4% nitroglycerin w/w (4mg... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Ointment |
| Route | Intra-anal |
| Strength | 0.4% |
| Market Status | Prescription |
| Company | Forest Labs |
| 8 of 14 | |
|---|---|
| Drug Name | Minitran |
| PubMed Health | Nitroglycerin |
| Drug Classes | Antianginal, Cardiovascular Agent, Colorectal Agent, Coronary Vasodilator, Nitrate |
| Drug Label | Nitroglycerin is a 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula isand whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.The MINITRAN (nitroglycerin) Transdermal... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 0.1mg/hr; 0.6mg/hr; 0.4mg/hr; 0.2mg/hr |
| Market Status | Prescription |
| Company | Valeant Pharm North; Medicis |
| 9 of 14 | |
|---|---|
| Drug Name | Nitro-dur |
| PubMed Health | Nitroglycerin Patch (Absorbed through the skin) |
| Drug Classes | Antianginal, Coronary Vasodilator |
| Drug Label | Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.The NITRO-DUR (nitroglycerin) Transdermal... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 0.1mg/hr; 0.6mg/hr; 0.4mg/hr; 0.2mg/hr; 0.8mg/hr; 0.3mg/hr |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 10 of 14 | |
|---|---|
| Drug Name | Nitroglycerin |
| PubMed Health | Nitroglycerin |
| Drug Classes | Antianginal, Cardiovascular Agent, Colorectal Agent, Coronary Vasodilator, Nitrate |
| Drug Label | Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.The NITRO-DUR (nitroglycerin) Transdermal... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Ointment; Spray, metered; Film, extended release; Injectable |
| Route | Injection; Sublingual; transdermal; Transdermal |
| Strength | 0.1mg/hr; 0.6mg/hr; 0.4mg/hr; 0.2mg/hr; 0.4mg/spray; 5mg/ml; 2% |
| Market Status | Tentative Approval; Prescription |
| Company | Noven; Hercon Pharm; Fougera; Luitpold; Mylan Technologies; Perrigo Israel |
| 11 of 14 | |
|---|---|
| Drug Name | Nitrolingual pumpspray |
| PubMed Health | Nitroglycerin |
| Drug Classes | Antianginal, Cardiovascular Agent, Colorectal Agent, Coronary Vasodilator, Nitrate |
| Drug Label | Nitroglycerin, an organic nitrate, is a vasodilator which has effects on both arteries and veins. The chemical name for nitroglycerin is 1,2,3-propanetriol trinitrate (C3H5N3O9). The compound has a molecular weight of 227.09. The chemical structure i... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Spray, metered |
| Route | Sublingual |
| Strength | 0.4mg/spray |
| Market Status | Prescription |
| Company | Pohl Boskamp |
| 12 of 14 | |
|---|---|
| Drug Name | Nitromist |
| Drug Label | Nitroglycerin, an organic nitrate, is a vasodilator which has effects on both arteries and veins. The chemical name for nitroglycerin is 1,2,3-propanetriol trinitrate (C3H5N3O9). The compound has a molecular weight of 227.09. The chemical structure i... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Aerosol, metered |
| Route | Sublingual |
| Strength | 0.4mg/spray |
| Market Status | Prescription |
| Company | Mist Pharms |
| 13 of 14 | |
|---|---|
| Drug Name | Nitrostat |
| Drug Label | NITROSTAT is a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg , or 0.6 mg nitroglycerin; as well as lactose monohydrate, NF; glyceryl monostearate, NF; pregelatinized starch, NF; calcium stearate, NF powder; and si... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Tablet |
| Route | Sublingual |
| Strength | 0.4mg; 0.6mg; 0.3mg |
| Market Status | Prescription |
| Company | Pfizer Pharms |
| 14 of 14 | |
|---|---|
| Drug Name | Rectiv |
| Drug Label | Nitroglycerin is 1,2,3,-propanetriol trinitrate, an organic nitrate whose structural formula is as follows:CH2-ONO2|CH-ONO2|CH2-ONO2and whose molecular weight is 227.09. RECTIV (nitroglycerin) Ointment 0.4% contains 0.4% nitroglycerin w/w (4mg... |
| Active Ingredient | Nitroglycerin |
| Dosage Form | Ointment |
| Route | Intra-anal |
| Strength | 0.4% |
| Market Status | Prescription |
| Company | Forest Labs |
Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Nitroglycerin can provide dramatic relief of paroxysmal nocturnal dyspnea, probably as result of improved left ventricular function and reduced pulmonary arterial pressure. ... Topical application of ... ointment may provide ... cutaneous vasodilation, particularly in treatment of raynaud's disease and ... healing of trophic ulcers.
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 809
MEDICATION (VET): In cardiotonic mixtures, to directly depress arterial muscles.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 391
MEDICATION (VET): Has been used for asthma in dogs.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1185
For more Therapeutic Uses (Complete) data for NITROGLYCERIN (17 total), please visit the HSDB record page.
Untoward responses to therapeutic use of nitrites are almost all secondary to actions on cardiovascular system. Headache is common ... Transient episodes of dizziness, weakness, and other manifestations of cerebral ischemia associated with postural hypotension may develop. ... In many pt, particularly if standing immobile, and may ... progress to loss of consciousness. This reaction appears to be accentuated by alcohol. ... methemoglobinemia. /organic nitrites/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 850
Contraindications: hypersensitivity to nitroglycerin or known idiosyncratic reaction to organic nitrates, hypotension or uncorrected hypovolemia, increased intracranial pressure, inadequate cerebral circulation, constrictive pericarditis, and pericardial tamponade.
Physicians Desk Reference p.1176-7 (1987)
Undesirable side effects from /nitroglycerin/ including: headaches, tachycardia, nausea, vomiting, apprehension, restlessness, muscle twitching, retrosternal discomfort, palpitations, dizziness, and abdominal pain. Paradoxical bradycardia and increased angina pectoris may accompany nitroglycerin-induced hypotension. ... With oral and/or topical use of nitroglycerin: cutaneous flushing, weakness, and occasionally, drug rash or exfoliative dermatitis.
Physicians Desk Reference p.1176-7 (1987)
In study groups, arterial partial pressure oxygen was decreased in most pt with coronary artery disease breathing room air in supine position. Clinical implications are discussed.
PMID:29482 KOPMAN ET AL; AM HEART J 96 (OCT): 444-7 (1978)
For more Drug Warnings (Complete) data for NITROGLYCERIN (14 total), please visit the HSDB record page.
Nitroglycerin is indicated for various purposes. It is indicated to prevent and treat angina or chest pain due to cardiovascular disease, as well as to treat peri-operative hypertension or induce intra-operative hypotension. It is also indicated to treat acute heart failure in patients with myocardial infarction. In the ointment form, nitroglycerin is indicated to treat pain caused by anal fissures. The transdermal form is applied directly to the skin to prevent acute anginal attacks. The intravenous form is used off-label in emergency settings and is commonly used to treat acute coronary spasm caused by cocaine, hypertensive emergencies, as well as acute congestive heart failure exacerbations. Some other off-label uses of nitroglycerin include management of variceal hemorrhage, management of esophageal spasticity, and induction of uterine relaxation.
Nitroglycerin causes the relaxation of vascular smooth muscles, causing arteriolar and venous dilatation. It reduces cardiac preload and afterload and reduces coronary artery spasm, decreasing systemic vascular resistance as well as systolic and diastolic blood pressure. The reduction of cardiac work by nitroglycerin is thought to cause the most relief of anginal symptoms, with some contributions from arteriolar dilatation effects.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Explosive Agents
Substances that are energetically unstable and can produce a sudden expansion of the material, called an explosion, which is accompanied by heat, pressure and noise. Other things which have been described as explosive that are not included here are explosive action of laser heating, human performance, sudden epidemiological outbreaks, or fast cell growth. (See all compounds classified as Explosive Agents.)
C01DA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DA - Organic nitrates
C01DA02 - Glyceryl trinitrate
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AE - Muscle relaxants
C05AE01 - Glyceryl trinitrate
Absorption
Nitroglycerin is rapidly absorbed and is often used in emergency situations for this reason. After a sublingual dose of 0.5 mg of nitroglycerin, peak concentration was reached by an average of in 4.4 minutes after administration and was measured to be 2.56 ng/ml. Cmax following a 0.6mg dose of sublingual nitroglycerin was measured to be 2.1 ng/mL and AUC was 14.9 minutes, and Tmax was 7.2 minutes. Absolute bioavailability after the administration of sublingual nitroglycerin tablets is about 40%. The bioavailability of nitroglycerin depends on factors such as mucosal metabolism and hydration status, which both affect the absorption of sublingual drugs.
Route of Elimination
Metabolism is the main route by which nitroglycerin is eliminated from the body.
Volume of Distribution
The volume of distribution of nitroglycerin is 3 L/kg.
Clearance
The FDA label for the intravenous form of nitroglycerin estimates clearance to be 1 L/kg/min. Apparent clearance after a sublingual dose was measured to be 21.9 L/min in a pharmacokinetic study of 22 patients with ischemic heart disease and angina.
In human beings, peak concentrations of nitroglycerin are found in plasma within 4 min of sublingual administration.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 849
volume of distribution 3.3 + or - 1.2 L/kg
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1990
Nitroglycerin is readily absorbed through the mucous membranes, lungs, and intact skin. An average plasma concentration of 2.3 ng/ml was observed one hour after dermal application of 45 mg of nitroglycerin as an ointment. Nitroglycerine is 60% bound to plasma protein at plasma concentrations between 50 and 500 ng/mL.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1378
The mono and di-nitrate metabolites are glucuronidated and excreted in the urine and bile.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1378
For more Absorption, Distribution and Excretion (Complete) data for NITROGLYCERIN (7 total), please visit the HSDB record page.
The enzyme mitchochondrial aldehyde dehydrogenase is known to cause the bioactivation of nitroglycerin. Nitroglycerin is metabolized to nitrite, 1,2-glyceryl dinitrate, and 1,3 glyceryl dinitrate. Nitrite is then metabolized to nitric oxide or S-nitrosothiol. The 1,2-and 1,3-dinitroglycerols are less potent in strength than nitroglycerin, but they have longer half-lives, explaining some prolonged effects of nitrates. Both dinitrates are subsequently metabolized to mononitrates that are not active on the blood vessels, and to glycerol and carbon dioxide in the final step of metabolism.
... Glyceryl ... /mononitrate is/ major circulating ... /metabolite/ of nitroglycerin.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 848
The metabolism of nitroglycerin... appears to involve reduced glutathione in a reaction catalyzed by an enzyme known as organic nitrate reductase, which is predominantly located in the soluble fraction of liver homogenate.
Zenz, C., O.B. Dickerson, E.P. Horvath. Occupational Medicine. 3rd ed. St. Louis, MO., 1994, p. 683
Nitroglycerin is degraded in the liver by reductive hydrolysis and partially in plasma by spontaneous hydrolysis... The major urinary metabolites include glyceryl mononitrate, 1,2-glyceryl dinitrate and 1,3-glyceryl dinitrate... Reactive hydrolysis in the liver by hepatic inorganic nitrate reductase leads to the formation of the free nitric oxide radical.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1378
Initial hydrolytic reduction by interaction of inorganic nitrate with reduced glutathione occurs both spontaneously and with enzymes, which can be extracted fron the soluble fraction of the liver.
Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 1018
For more Metabolism/Metabolites (Complete) data for NITROGLYCERIN (6 total), please visit the HSDB record page.
In a pharmacokinetic study, the plasma half-life of intravenously administered nitroglycerin was 2.8 0.9 minutes. The FDA label for the intravenous form of nitroglycerin indicates a similar plasma half-life of about 3 minutes. A pharmacokinetic study using sublingual nitroglycerin estimated the plasma half-life to be approximately 6 minutes. The elimination half-lives of 1,2- and 1,3-dinitroglycerin (metabolites of nitroglycerin)range between 32-26 minutes.
Half-life 2.3 + or - 0.6 minutes.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1990
The plasma half-life of nitroglycerin has been estimated at 1 to 3 minutes, representing the alpha distribution, and also at about 7.5 minutes.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1378
The elim half-life of 1,2- and 1,3-dinitroglycerin is 36 and 32 min, respectively.
Medical Economics Co; Physicians Desk Reference 56th ed p.2659 (2002)
Nitroglycerin is converted by mitochondrial aldehyde dehydrogenase (mtALDH) to nitric oxide (NO), an active substance which then activates the enzyme guanylate cyclase. The activation of this enzyme is followed by the synthesis of cyclic guanosine 3',5'-monophosphate (cGMP), activating a cascade of protein kinase-dependent phosphorylation events in smooth muscles. This process eventually leads to the dephosphorylation of the myosin light chain of smooth muscles, causing relaxation and increased blood flow in veins, arteries and cardiac tissue.. The above processes lead to decreased work of the heart decreased blood pressure, relief of anginal symptoms, and increased blood flow to the myocardium.One in vitro study using mouse aorta suggests that nitric oxide (an activated metabolite of nitroglycerin) targets the natriuretic peptide receptors.
The drugs used to treat angina alleviate symptoms by increasing blood flow to the ischemic myocardium and/or by reducing myocardial oxygen requirements. ... /Nitrates/ reduce myocardial oxygen requirements through their effects on the systemic circulation. Their major systemic action is a reduction in venous tone, which leads to pooling of blood in peripheral veins, decreased venous return, and reduced ventricular volume and myocardial tension (preload). /Org nitrates/
American Medical Association, Department of Drugs. Drug Evaluations. 6th ed. Chicago, Ill: American Medical Association, 1986., p. 463
Although it predominately affects vascular smooth muscle /nitroglycerin/, the bronchioles, gastrointestinal tract (including biliary system), ureters, and uterus are affected. Free radicals of nitric oxide may activate guanylate cyclase, resulting in increased synthesis of cyclic GMP. Nitric oxide may combine with sulfhydryl groups in the endothelium and produce S-nitrosothiols, which stimulate guanylate cyclase production. N-acetyl-cycteine may enhance this process by providing a source of sulfhydryl groups. Cyclic GMP appears to reduce stored calcium and interfere with calcium-activated smooth muscle contractions.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1378
Organic nitrates... lead to the formation of the reactive free radical nitric oxide, which can activate guanylyl cyclase and increase the synthesis of cyclic GMP in smooth muscle and other tissues... A cyclic GMP-dependent protein kinase catalyzes the phosphorylation of various proteins in smooth muscle. Eventually, the light chain of myosin is dephosphorylated. Phosphorylation of the myosin chain regulates the maintenance of the contractile state in smooth muscle.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 848
Nitrates also may alter the prostaglandin system by inhibiting thromboxane synthetase and permitting preferential formation of prostacyclin over thromboxane A2. Both of these two short-acting vasoactive substances are formed from prostaglandin precursors. Prostacyclin is a potent vasodilator which causes smooth muscle relaxation through phosphorylation of the myosin light chain kinase. This reduces its ability to to be activated by calciun and calmodulin.
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 13-7
GDUFA
DMF Review : Complete
Rev. Date : 2022-08-01
Pay. Date : 2022-07-27
DMF Number : 4313
Submission : 1981-11-03
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-10-15
Pay. Date : 2013-03-12
DMF Number : 1839
Submission : 1971-12-16
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-06-24
Pay. Date : 2014-10-09
DMF Number : 3731
Submission : 1980-02-07
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 1923
Submission : 1972-05-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4171
Submission : 1981-04-06
Status : Inactive
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5254
Submission : 1984-01-16
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 1040
Submission : 1967-03-07
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5238
Submission : 1984-02-16
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5178
Submission : 1983-11-30
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2003-185 - Rev 02
Status : Valid
Issue Date : 2025-03-11
Type : Chemical
Substance Number : 1331

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2008-285 - Rev 00
Status : Valid
Issue Date : 2014-05-19
Type : TSE
Substance Number :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
32
PharmaCompass offers a list of Nitroglycerin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nitroglycerin manufacturer or Nitroglycerin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nitroglycerin manufacturer or Nitroglycerin supplier.
PharmaCompass also assists you with knowing the Nitroglycerin API Price utilized in the formulation of products. Nitroglycerin API Price is not always fixed or binding as the Nitroglycerin Price is obtained through a variety of data sources. The Nitroglycerin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nitroglycerin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nitroglycerin, including repackagers and relabelers. The FDA regulates Nitroglycerin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nitroglycerin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nitroglycerin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nitroglycerin supplier is an individual or a company that provides Nitroglycerin active pharmaceutical ingredient (API) or Nitroglycerin finished formulations upon request. The Nitroglycerin suppliers may include Nitroglycerin API manufacturers, exporters, distributors and traders.
click here to find a list of Nitroglycerin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nitroglycerin DMF (Drug Master File) is a document detailing the whole manufacturing process of Nitroglycerin active pharmaceutical ingredient (API) in detail. Different forms of Nitroglycerin DMFs exist exist since differing nations have different regulations, such as Nitroglycerin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nitroglycerin DMF submitted to regulatory agencies in the US is known as a USDMF. Nitroglycerin USDMF includes data on Nitroglycerin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nitroglycerin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nitroglycerin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nitroglycerin Drug Master File in Japan (Nitroglycerin JDMF) empowers Nitroglycerin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nitroglycerin JDMF during the approval evaluation for pharmaceutical products. At the time of Nitroglycerin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nitroglycerin suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Nitroglycerin Drug Master File in Korea (Nitroglycerin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Nitroglycerin. The MFDS reviews the Nitroglycerin KDMF as part of the drug registration process and uses the information provided in the Nitroglycerin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Nitroglycerin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Nitroglycerin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Nitroglycerin suppliers with KDMF on PharmaCompass.
A Nitroglycerin CEP of the European Pharmacopoeia monograph is often referred to as a Nitroglycerin Certificate of Suitability (COS). The purpose of a Nitroglycerin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Nitroglycerin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Nitroglycerin to their clients by showing that a Nitroglycerin CEP has been issued for it. The manufacturer submits a Nitroglycerin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Nitroglycerin CEP holder for the record. Additionally, the data presented in the Nitroglycerin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Nitroglycerin DMF.
A Nitroglycerin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Nitroglycerin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Nitroglycerin suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nitroglycerin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nitroglycerin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nitroglycerin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nitroglycerin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nitroglycerin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nitroglycerin suppliers with NDC on PharmaCompass.
Nitroglycerin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nitroglycerin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nitroglycerin GMP manufacturer or Nitroglycerin GMP API supplier for your needs.
A Nitroglycerin CoA (Certificate of Analysis) is a formal document that attests to Nitroglycerin's compliance with Nitroglycerin specifications and serves as a tool for batch-level quality control.
Nitroglycerin CoA mostly includes findings from lab analyses of a specific batch. For each Nitroglycerin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nitroglycerin may be tested according to a variety of international standards, such as European Pharmacopoeia (Nitroglycerin EP), Nitroglycerin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nitroglycerin USP).

