
Synopsis
Synopsis
0
VMF
0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
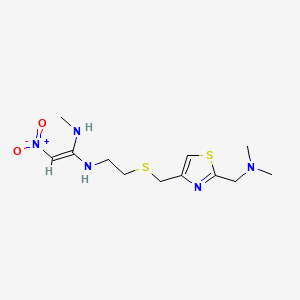

1. Axid
2. Ly 139037
3. Ly-139037
4. Ly139037
5. N-(2-(((2-((dimethylamino)methyl)-4-thiazolyl)methyl)thio)ethyl)-n'-methyl-2-nitro-1,1-ethenediamine
1. 76963-41-2
2. Axid
3. Zanizal
4. Nizax
5. Ly-139037
6. Niaztidine
7. Nizatidinum
8. Nizatidina
9. Ze-101
10. Zl-101
11. (e)-n-(2-(((2-((dimethylamino)methyl)thiazol-4-yl)methyl)thio)ethyl)-n'-methyl-2-nitroethene-1,1-diamine
12. (e)-n-(2-(((2-((dimethylamino)methyl)thiazol-4-yl)methyl)thio)ethyl)-n-methyl-2-nitroethene-1,1-diamine
13. Dimethyl[(4-{[(2-{[(e)-1-(methylamino)-2-nitroethenyl]amino}ethyl)sulfanyl]methyl}-1,3-thiazol-2-yl)methyl]amine
14. N-(4-(6-methylamino-7-nitro-2-thia-5-aza-6-hepten-1-yl)-2-thiazolylmethyl)-n,n-dimethylamine
15. Smr000466384
16. Acinon (tn)
17. Axid (tn)
18. Chebi:7601
19. Mfcd00865660
20. Ncgc00016934-01
21. Cas-76963-41-2
22. Prestwick2_000921
23. Schembl769
24. Schembl770
25. Chembl653
26. Ly-139037, Nizatidine
27. Mls000759518
28. Mls001076680
29. Mls001424001
30. Bidd:gt0761
31. Nizatidine, Analytical Standard
32. Gtpl7248
33. Nizatidine (jp17/usp/inn)
34. Nizatidine For System Suitability
35. Hms2051k04
36. Hms2094a15
37. Hms2235n05
38. Pharmakon1600-01505985
39. Hy-b0310
40. Zinc1530736
41. Nsc759289
42. Akos015900643
43. Ac-5272
44. Ccg-100836
45. Db00585
46. Hs-0083
47. Nc00086
48. Ncgc00016934-02
49. (e)-n-{2-[({2-[(dimethylamino)methyl]-1,3-thiazol-4-yl}methyl)thio]ethyl}-n'-methyl-2-nitroethene-1,1-diamine
50. Bn166185
51. Sbi-0206937.p001
52. C07270
53. D00440
54. Ab00698253-07
55. Ab00698253_09
56. Benzenesulfonylchloride,4-hydroxy-3-nitro-(9ci)
57. 963n412
58. A838919
59. L000761
60. Sr-01000765410
61. Q1188290
62. Sr-01000765410-4
63. Brd-k73589491-001-05-4
64. Brd-k92193792-001-01-7
65. (e)-1-n'-[2-[[2-[(dimethylamino)methyl]-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-1-n-methyl-2-nitroethene-1,1-diamine
66. (e)-n-(2-((2-((dimethylamino)methyl)thiazol-4-yl)methylthio)ethyl)-n-methyl-2-nitroethene-1,1-diamine
67. (e)-n1'-[2-[[2-[(dimethylamino)methyl]-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-n1-methyl-2-nitro-ethene-1,1-diamine
68. (e)-n1'-[2-[[2-[(dimethylamino)methyl]-4-thiazolyl]methylthio]ethyl]-n1-methyl-2-nitroethene-1,1-diamine
69. 1,1-ethenediamine, N'-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-n-methyl-2-nitro-
70. N-(2-(((2-((dimethylamino)methyl)thiazol-4-yl)methyl)thio)ethyl)-n-methyl-2-nitroethene-1,1-diamine
71. N-{2-[({2-[(dimethylamino)methyl]-1,3-thiazol-4-yl}methyl)sulfanyl]ethyl}-n'-methyl-2-nitroethene-1,1-diamine
| Molecular Weight | 331.5 g/mol |
|---|---|
| Molecular Formula | C12H21N5O2S2 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 331.11366728 g/mol |
| Monoisotopic Mass | 331.11366728 g/mol |
| Topological Polar Surface Area | 140 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 349 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Axid |
| PubMed Health | Nizatidine (By mouth) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | Nizatidine (USP) is a histamine H2-receptor antagonist. Chemically, it is N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine. The structural formula is as follows: Nizatidine has the empirical formula |
| Active Ingredient | Nizatidine |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 15mg/ml |
| Market Status | Prescription |
| Company | Braintree |
| 2 of 6 | |
|---|---|
| Drug Name | Axid ar |
| Active Ingredient | Nizatidine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 75mg |
| Market Status | Over the Counter |
| Company | Pfizer |
| 3 of 6 | |
|---|---|
| Drug Name | Nizatidine |
| PubMed Health | Nizatidine (By mouth) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | Nizatidine USP is a histamine H2-receptor antagonist. Chemically, it is N-[2-[[[2-[(Dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine. The structural formula is represented below:C12H21N5O2S2... |
| Active Ingredient | Nizatidine |
| Dosage Form | Capsule; Solution |
| Route | Oral |
| Strength | 150mg; 300mg; 15mg/ml |
| Market Status | Prescription |
| Company | Amneal Pharms; Mylan Pharms; Ani Pharms; Sandoz; Watson Labs; Glenmark Generics; Dr Reddys Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Axid |
| PubMed Health | Nizatidine (By mouth) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | Nizatidine (USP) is a histamine H2-receptor antagonist. Chemically, it is N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine. The structural formula is as follows: Nizatidine has the empirical formula |
| Active Ingredient | Nizatidine |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 15mg/ml |
| Market Status | Prescription |
| Company | Braintree |
| 5 of 6 | |
|---|---|
| Drug Name | Axid ar |
| Active Ingredient | Nizatidine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 75mg |
| Market Status | Over the Counter |
| Company | Pfizer |
| 6 of 6 | |
|---|---|
| Drug Name | Nizatidine |
| PubMed Health | Nizatidine (By mouth) |
| Drug Classes | Antiulcer, Gastric Acid Secretion Inhibitor |
| Drug Label | Nizatidine USP is a histamine H2-receptor antagonist. Chemically, it is N-[2-[[[2-[(Dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine. The structural formula is represented below:C12H21N5O2S2... |
| Active Ingredient | Nizatidine |
| Dosage Form | Capsule; Solution |
| Route | Oral |
| Strength | 150mg; 300mg; 15mg/ml |
| Market Status | Prescription |
| Company | Amneal Pharms; Mylan Pharms; Ani Pharms; Sandoz; Watson Labs; Glenmark Generics; Dr Reddys Labs |
Anti-Ulcer Agents; Histamine H2 Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
In the Zollinger-Ellison syndrome and other acid peptic disorders, the efficacy of nizatidine would be expected to be similar to that of ranitidine, but confirmation in clinical studies is required. Nizatidine relieves symptoms and heals lesions in patients with mild to moderate reflux esophagitis.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 903
Histamine H2-receptor antagonists are indicated in the short-term treatment of active duodenal ulcer. They are also indicated (at reduced dosage) in the prevention of duodenal ulcer recurrence in selected patients. /Histamine H2-receptor antagonists; Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1611
Nizatidine ... /is/ indicated in the short-term treatment of active benign gastric ulcer. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1611
For more Therapeutic Uses (Complete) data for NIZATIDINE (8 total), please visit the HSDB record page.
Nizatidine should be used with caution and the dose and/or frequency of administration reduced in patients with renal impairment (ie., creatinine clearance less than 50 ml/min), since the drug is excreted principally by the kidneys.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2174
Symptomatic response to nizatidine should not be interpreted as precluding the presence of gastric malignancy.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2174
Rhinitis, pharyngitis, sinusitis, or increased cough has occurred in about 2-10% of patients receiving nizatidine. Diaphoresis occurred more often with nizatidine therapy than with placebo but was not considered drug related because of its association with other concurrent adverse events (e.g., fever, anxiety). Pain, including back or chest pain, has been reported in approximately 2-4% of patients receiving nizatidine. Other adverse events reported in patients receiving nizatidine include myalgia, fever, infection, amblyopia, hyperuricemia, and impotence. In most studies, the incidence of these adverse effects, and of surgical procedures or accidental injuries, was similar in patients receiving nizatidine or placebo. Asymptomatic ventricular tachycardia, decreased libido, and gynecomastia have been reported rarely.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2174
Urticaria occurred more often with nizatidine therapy (0.5% of patients) than with placebo (0.1% of patients) in placebo-controlled clinical studies. Rash, pruritus, or exfoliative dermatitis has occurred in up to 2% of patients receiving nizatidine. Serum sickness-like reactions, anaphylaxis, bronchospasm, laryngeal edema, eosinophilia, vasculitis, and erythema multiforme also have been reported rarely.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2174
For more Drug Warnings (Complete) data for NIZATIDINE (7 total), please visit the HSDB record page.
For the treatment of acid-reflux disorders (GERD), peptic ulcer disease, active benign gastric ulcer, and active duodenal ulcer.
FDA Label
Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H2-receptors, particularly those in the gastric parietal cells. By inhibiting the action of histamine on stomach cells, nizatidine reduces stomach acid production. Nizatidine had no demonstrable antiandrogenic action. Full-dose therapy for the problems treated by nizatidine lasts no longer than 8 weeks. It has been demonstrated that treatment with a reduced dose of nizatidine is effective as maintenance therapy following healing of active duodenal ulcers.
Histamine H2 Antagonists
Drugs that selectively bind to but do not activate histamine H2 receptors, thereby blocking the actions of histamine. Their clinically most important action is the inhibition of acid secretion in the treatment of gastrointestinal ulcers. Smooth muscle may also be affected. Some drugs in this class have strong effects in the central nervous system, but these actions are not well understood. (See all compounds classified as Histamine H2 Antagonists.)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BA - H2-receptor antagonists
A02BA04 - Nizatidine
Absorption
Rapid (bioavailability of nizatidine exceeds 70%)
Volume of Distribution
0.8 to 1.5 L/kg
Clearance
40-60 L/h
7 14 L/h [functionally anephric patients]
Nizatidine has a duration of action of up to 10 hours. It is eliminated primarily by the kidneys; 90% of the administered dose (65% as unchanged drug) is recovered in the urine within 16 hours.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 904
Distribution of nizatidine into human body tissues and fluids has not been fully characterized. The apparent volume of distribution of the drug is reported to be 0.8-1.5 l/kg in adults and does not appear to be altered substantially in patients with renal dysfunction.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2172
In one study, the oral bioavailability of nizatidine exceeded 70% and was not affected by food or the anticholinergic drug, propantheline. Its apparent volume of distribution is 1.2 l/kg. Systemic clearance (10 ml/min/kg) is decreased in uremic patients and in the elderly.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 904
The safety and disposition of single oral doses of nizatidine were investigated in 8 young volunteers (aged 25-48 yr) who received 100-350 mg nizatidine and in 12 elderly volunteers (aged 66-79 yr) who received 100-300 mg. The nizatidine AUC was directly proportional to dose for both groups of volunteers. Calculated pharmacokinetic variables in the elderly versus the young were Tl/2 (1.9 versus 1.6 hr), apparent plasma clearance (32 versus 40 l/h) and apparent volume of distribution (1.2 versus 1.3 l/kg). The impaired renal function of some elderly volunteers prolonged nizatidine elimination and lowered its clearance. Renal impairment rather than advanced age per se was the predominant factor in decreasing the nizatidine elimination rate. No serious adverse effects occurred.
PMID:2888796 Callaghan JT et al; J Clin Pharmacol 27 (Aug): 618-24 (1987)
For more Absorption, Distribution and Excretion (Complete) data for NIZATIDINE (7 total), please visit the HSDB record page.
Hepatic. Less than 7% of an oral dose is metabolized as N2-monodes-methylnizatidine, an H2-receptor antagonist, which is the principal metabolite excreted in the urine. Other likely metabolites are the N2-oxide (less than 5% of the dose) and the S-oxide (less than 6% of the dose).
Nizatidine is metabolized in the liver to N-desmethylnizatidine, nizatidine N-oxide, and nizatidine sulfoxide. Of these metabolites, only N-desmethylnizatidine has histamine H2-receptor blocking activity; studies in animals indicate that this metabolite is approximately 60% as active as nizatidine in blocking gastric acid secretion. Orally administered nizatidine undergoes minimal metabolism on first pass through the liver.
McEvoy G.K. (ed.). American Hospital Formulary Service-Drug Information 96. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1996 (Plus Supplements)., p. 2172
1-2 hours
Half life is 10 hours.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 904
Nizatidine competes with histamine for binding at the H2-receptors on the gastric basolateral membrane of parietal cells. Competitive inhibition results in reduction of basal and nocturnal gastric acid secretions. The drug also decreases the gastric acid response to stimuli such as food, caffeine, insulin, betazole, or pentagastrin.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
24
PharmaCompass offers a list of Nizatidine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nizatidine manufacturer or Nizatidine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nizatidine manufacturer or Nizatidine supplier.
PharmaCompass also assists you with knowing the Nizatidine API Price utilized in the formulation of products. Nizatidine API Price is not always fixed or binding as the Nizatidine Price is obtained through a variety of data sources. The Nizatidine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nizatidine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nizatidine, including repackagers and relabelers. The FDA regulates Nizatidine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nizatidine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nizatidine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nizatidine supplier is an individual or a company that provides Nizatidine active pharmaceutical ingredient (API) or Nizatidine finished formulations upon request. The Nizatidine suppliers may include Nizatidine API manufacturers, exporters, distributors and traders.
click here to find a list of Nizatidine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nizatidine DMF (Drug Master File) is a document detailing the whole manufacturing process of Nizatidine active pharmaceutical ingredient (API) in detail. Different forms of Nizatidine DMFs exist exist since differing nations have different regulations, such as Nizatidine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nizatidine DMF submitted to regulatory agencies in the US is known as a USDMF. Nizatidine USDMF includes data on Nizatidine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nizatidine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nizatidine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nizatidine Drug Master File in Japan (Nizatidine JDMF) empowers Nizatidine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nizatidine JDMF during the approval evaluation for pharmaceutical products. At the time of Nizatidine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nizatidine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Nizatidine Drug Master File in Korea (Nizatidine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Nizatidine. The MFDS reviews the Nizatidine KDMF as part of the drug registration process and uses the information provided in the Nizatidine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Nizatidine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Nizatidine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Nizatidine suppliers with KDMF on PharmaCompass.
A Nizatidine CEP of the European Pharmacopoeia monograph is often referred to as a Nizatidine Certificate of Suitability (COS). The purpose of a Nizatidine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Nizatidine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Nizatidine to their clients by showing that a Nizatidine CEP has been issued for it. The manufacturer submits a Nizatidine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Nizatidine CEP holder for the record. Additionally, the data presented in the Nizatidine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Nizatidine DMF.
A Nizatidine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Nizatidine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Nizatidine suppliers with CEP (COS) on PharmaCompass.
A Nizatidine written confirmation (Nizatidine WC) is an official document issued by a regulatory agency to a Nizatidine manufacturer, verifying that the manufacturing facility of a Nizatidine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Nizatidine APIs or Nizatidine finished pharmaceutical products to another nation, regulatory agencies frequently require a Nizatidine WC (written confirmation) as part of the regulatory process.
click here to find a list of Nizatidine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nizatidine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nizatidine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nizatidine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nizatidine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nizatidine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nizatidine suppliers with NDC on PharmaCompass.
Nizatidine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nizatidine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nizatidine GMP manufacturer or Nizatidine GMP API supplier for your needs.
A Nizatidine CoA (Certificate of Analysis) is a formal document that attests to Nizatidine's compliance with Nizatidine specifications and serves as a tool for batch-level quality control.
Nizatidine CoA mostly includes findings from lab analyses of a specific batch. For each Nizatidine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nizatidine may be tested according to a variety of international standards, such as European Pharmacopoeia (Nizatidine EP), Nizatidine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nizatidine USP).
