
Synopsis
Synopsis
0
USDMF
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Arterenol
2. Levarterenol
3. Levonor
4. Levonorepinephrine
5. Levophed
6. Levophed Bitartrate
7. Noradrnaline Tartrate Renaudin
8. Noradrenaline
9. Noradrenaline Bitartrate
10. Norepinephrin D-tartrate (1:1)
11. Norepinephrine Bitartrate
12. Norepinephrine D-tartrate (1:1)
13. Norepinephrine Hydrochloride
14. Norepinephrine Hydrochloride, (+)-isomer
15. Norepinephrine Hydrochloride, (+,-)-isomer
16. Norepinephrine L-tartrate (1:1)
17. Norepinephrine L-tartrate (1:1), (+,-)-isomer
18. Norepinephrine L-tartrate (1:1), Monohydrate
19. Norepinephrine L-tartrate (1:1), Monohydrate, (+)-isomer
20. Norepinephrine L-tartrate (1:2)
21. Norepinephrine L-tartrate, (+)-isomer
22. Norepinephrine, (+)-isomer
23. Norepinephrine, (+,-)-isomer
1. Noradrenaline
2. L-noradrenaline
3. 51-41-2
4. Arterenol
5. Levarterenol
6. (r)-noradrenaline
7. Levophed
8. (-)-norepinephrine
9. L-norepinephrine
10. (-)-noradrenaline
11. (r)-norepinephrine
12. Levonor
13. L-arterenol
14. 4-[(1r)-2-amino-1-hydroxyethyl]benzene-1,2-diol
15. (-)-arterenol
16. Aktamin
17. Levonoradrenaline
18. Levonorepinephrine
19. Adrenor
20. Sympathin E
21. (r)-(-)-norepinephrine
22. Noradrenalin
23. Norepirenamine
24. Nor-epirenan
25. Norepinephrinum
26. Levoarterenol
27. (r)-4-(2-amino-1-hydroxyethyl)-1,2-benzenediol
28. Norartrinal
29. Nor Adrenalin
30. L-3,4-dihydroxyphenylethanolamine
31. L-1-(3,4-dihydroxyphenyl)-2-aminoethanol
32. L-2-amino-1-(3,4-dihydroxyphenyl)ethanol
33. (-)-(r)-norepinephrine
34. (-)-alpha-(aminomethyl)protocatechuyl Alcohol
35. Nor Adrenalin (tn)
36. L-alpha-(aminomethyl)-3,4-dihydroxybenzyl Alcohol
37. 4-[(1r)-2-amino-1-hydroxyethyl]-1,2-benzenediol
38. 1,2-benzenediol, 4-[(1r)-2-amino-1-hydroxyethyl]-
39. Noradrenalina
40. 1,2-benzenediol, 4-(2-amino-1-hydroxyethyl)-, (r)-
41. Norepinephrine (inn)
42. X4w3enh1cv
43. Nsc-757246
44. Chembl1437
45. 51-41-2 (free Base)
46. Benzyl Alcohol, Alpha-(aminomethyl)-3,4-dihydroxy-, (-)-
47. Noradrenalinum
48. Chebi:18357
49. 4-[(1r)-2-amino-1-hydroxy-ethyl]benzene-1,2-diol
50. Levarterenolo
51. Noreinefrina
52. Levarterenolo [dcit]
53. D-(-)-noradrenaline
54. Noradrenalina [italian]
55. Noradrenalin, L-
56. Norepinephrine [inn]
57. 65277-62-5
58. D-arterenol
59. Noreinefrina [inn-spanish]
60. Norepinephrinum [inn-latin]
61. Norepinefrina
62. Ecteinascidins
63. (+)-noradrenaline
64. 1,2-benzenediol, 4-[(1r)-2-amino-1-hydroxyethyl]-, Homopolymer
65. (?)-norepinephrine
66. Noradrenaline (jp15)
67. Einecs 200-096-6
68. Unii-x4w3enh1cv
69. (-)-arterenol Free Base
70. Norepinephrine Noradrenalin
71. 1,2-benzenediol, 4-((1r)-2-amino-1-hydroxyethyl)-
72. Brn 4231961
73. Nor-adrenaline
74. R-norepinephrine
75. Dermx
76. Norepinephrine [inn:ban:jan]
77. Hsdb 7772
78. E5e
79. (r)-4-(2-amino-1-hydroxyethyl)benzene-1,2-diol
80. Albb-006229
81. Norepinephirine
82. Spectrum_001009
83. Noradrenaline (jp17)
84. Spectrum2_001064
85. Spectrum3_000520
86. Spectrum4_000078
87. Spectrum5_001068
88. Dsstox_cid_3378
89. Bmse000404
90. 1,2-benzenediol,4-(2-amino-1-hydroxyethyl)-
91. Noradrenaline [jan]
92. Norepinephrine [mi]
93. Schembl2609
94. Dsstox_rid_77004
95. Dsstox_gsid_23378
96. Bspbio_002079
97. Gtpl505
98. Kbiogr_000635
99. Kbioss_001489
100. Norepinephrine [hsdb]
101. 4-(2-amino-1-hydroxyethyl)-1,2-benzenediol
102. Mls006010883
103. Divk1c_000230
104. Noradrenaline [mart.]
105. Norepinephrine [vandf]
106. Spectrum1500436
107. Spbio_001048
108. Levarterenol;levophed;arterenol
109. 1,2-benzenediol, 4-((r)-2-amino-1-hydroxyethyl)-
110. Sgcut00123
111. Norepinephrine [who-dd]
112. Dtxsid5023378
113. Hms500l12
114. Kbio1_000230
115. Kbio2_001489
116. Kbio2_004057
117. Kbio2_006625
118. Kbio3_001579
119. Zinc57624
120. D53d5e3a-2360-4ca9-8031-6c2cd4062fd5
121. Ninds_000230
122. Hms1920b08
123. Hms2089e18
124. Hms2091j08
125. Hms3887i07
126. Pharmakon1600-01500436
127. To_000024
128. Tox21_301944
129. Bdbm50029051
130. Ccg-40104
131. Mfcd00025592
132. Norepinephrine [usp Impurity]
133. Nsc757246
134. Pdsp1_001111
135. Pdsp2_001095
136. S9507
137. Stk503776
138. Akos006345192
139. Akos015854572
140. Db00368
141. Nsc 757246
142. Sdccgsbi-0050082.p004
143. Cas-51-41-2
144. Idi1_000230
145. Ncgc00159406-03
146. Ncgc00159406-04
147. Ncgc00159406-05
148. Ncgc00159406-06
149. Ncgc00159406-07
150. Ncgc00159406-08
151. Ncgc00159406-09
152. Ncgc00159406-10
153. Ncgc00159406-11
154. Ncgc00159406-17
155. Ncgc00255328-01
156. As-56654
157. Hy-13715
158. Smr000058585
159. (-)-norepinephrine, >=98%, Crystalline
160. (r)-4-(2-amino-1-hydroxyethyl)catechol
161. Sbi-0050082.p003
162. Adrenaline Impurity B [ep Impurity]
163. Cs-0007744
164. C00547
165. D00076
166. Ab00052424-06
167. Ab00052424_07
168. Ab00052424_08
169. 025n592
170. Q186242
171. Adrenaline Tartrate Impurity B [ep Impurity]
172. W-105896
173. (1r)-2-amino-1-(3,4-dihydroxyphenyl)ethanol
174. (-)-.alpha.-(aminomethyl)-3,4-dihydroxybenzyl Alcohol
175. 1,2-benzenediol, 4-(2-amino-1-hydroxyethyl)-, (r)-(-)-
176. Benzyl Alcohol, .alpha.-(aminomethyl)-3,4-dihydroxy-, (+)-
177. (-)-noradrenaline/ 1,?2-?benzenediol, 4-?[(1r)?-?2-?amino-?1-?hydroxyethyl]?-
178. 1,2-benzenediol, 4-(2-amino-1-hydroxyethyl)-, (r)-(r-(r*,r*))-2,3-dihydroxybutanedioate
179. 86941-27-7
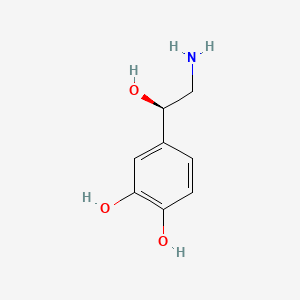
| Molecular Weight | 169.18 g/mol |
|---|---|
| Molecular Formula | C8H11NO3 |
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 169.07389321 g/mol |
| Monoisotopic Mass | 169.07389321 g/mol |
| Topological Polar Surface Area | 86.7 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 142 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Norepinephrine is used to produce vasoconstriction and cardiac stimulation as an adjunct to correct hemodynamic imbalances in the treatment of shock that persists after adequate fluid volume replacement. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1397
Epinephrine is the drug of choice in the emergency treatment of severe acute anaphylactic reactions, including anaphylactic shock. Once adequate ventilation is assured, maintenance of blood pressure in patients with anaphylactic shock may be achieved with other pressor agents, such as norepinephrine. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1397
In hypotension associated with myocardial infarction, cautious administration of norepinephrine may be of value and some clinicians consider it to be the pressor drug of choice. However, this type of shock generally has a poor prognosis even when pressor agents are used, and norepinephrine-induced increases in myocardial oxygen demand and the work of the heart may outweigh the beneficial effects of the drug. In addition, cardiac arrhythmias due to the drug are more likely to occur in patients with myocardial infarction. If severe congestive heart failure is also present, dopamine may be preferable because it increases renal blood flow as well as stroke volume. If peripheral vascular resistance is elevated, isoproterenol may be used in conjunction with norepinephrine, but dosage of both drugs must be carefully adjusted according to the specific hemodynamic imbalances present. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1397
Norepinephrine may be used to treat hypotension occurring during spinal anesthesia, but other vasopressors having a longer duration of action and which can be administered IM such as metaraminol, methoxamine, or phenylephrine are more commonly used. Norepinephrine may be used to treat hypotension occurring during general anesthesia; however, the possibility of cardiac arrhythmias should be considered. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1398
For more Therapeutic Uses (Complete) data for Norepinephrine (7 total), please visit the HSDB record page.
Norepinephrine can cause severe peripheral and visceral vasoconstriction, reduced blood flow to vital organs, decreased renal perfusion and therefore decreased urine output, tissue hypoxia, and metabolic acidosis. These effects are most likely to occur in hypovolemic patients. In addition, prolonged use of norepinephrine may cause plasma volume depletion which may result in perpetuation of the shock state or recurrence of hypotension when the drug is discontinued.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1398
Prolonged administration of norepinephrine has caused edema, hemorrhage, focal myocarditis, subpericardial hemorrhage, necrosis of the intestine, or hepatic and renal necrosis. These effects have generally occurred in patients with severe shock and it is not clear if the drug or the shock state itself was the cause.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1399
Norepinephrine can cause tissue necrosis and sloughing at the site of injection as a result of local vasoconstriction. Impairment of circulation and sloughing of tissue may also occur without obvious extravasation. Gangrene of the extremities has been reported rarely and has occurred in a lower extremity when norepinephrine was injected into an ankle vein.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1398
Norepinephrine increases myocardial oxygen consumption and the work of the heart. Cardiac output may be decreased following prolonged use of the drug or administration of large doses because venous return to the heart may be diminished because of increased peripheral vascular resistance. Decreased cardiac output may be especially harmful to elderly patients or those with initially poor cerebral or coronary circulation. Norepinephrine may cause palpitation and bradycardia as well as potentially fatal cardiac arrhythmias, including ventricular tachycardia, bigeminal rhythm, nodal rhythm, atrioventricular dissociation, and fibrillation. Bradycardia may be treated by administration of atropine. Arrhythmias are especially likely to occur in patients with acute myocardial infarction, hypoxia, or hypercapnia, or those receiving other drugs which may increase cardiac irritability such as cyclopropane or halogenated hydrocarbon general anesthetics.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1398
For more Drug Warnings (Complete) data for Norepinephrine (19 total), please visit the HSDB record page.
Mainly used to treat patients in vasodilatory shock states such as septic shock and neurogenic shock and has shown a survival benefit over dopamine. Also used as a vasopressor medication for patients with critical hypotension.
Noradrenaline acts on both alpha-1 and alpha-2 adrenergic receptors to cause vasoconstriction. Its effect in-vitro is often limited to the increasing of blood pressure through antagonising alpha-1 and alpha-2 receptors and causing a resultant increase in systemic vascular resistance.
Adrenergic alpha-Agonists
Drugs that selectively bind to and activate alpha adrenergic receptors. (See all compounds classified as Adrenergic alpha-Agonists.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
C01CA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01C - Cardiac stimulants excl. cardiac glycosides
C01CA - Adrenergic and dopaminergic agents
C01CA03 - Norepinephrine
Norepinephrine localizes mainly in sympathetic nervous tissue. The drug crosses the placenta but not the blood-brain barrier.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1400
Orally ingested norepinephrine is destroyed in the GI tract, and the drug is poorly absorbed after subcutaneous injection. After IV administration, a pressor response occurs rapidly. The drug has a short duration of action, and the pressor action stops within 1-2 minutes after the infusion is discontinued.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1400
Norepinephrine, like epinephrine, is ineffective when given orally and is absorbed poorly from sites of subcutaneous injection. It is rapidly inactivated in the body by the same enzymes that methylate and oxidatively deaminate epinephrine. Small amounts normally are found in the urine. The excretion rate may be greatly increased in patients with pheochromocytoma.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 248
The pharmacologic actions of norepinephrine are terminated primarily by uptake and metabolism in sympathetic nerve endings. The drug is metabolized in the liver and other tissues by a combination of reactions involving the enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). The major metabolites are normetanephrine and 3-methoxy-4-hydroxy mandelic acid (vanillylmandelic acid, VMA), both of which are inactive. Other inactive metabolites include 3-methoxy-4-hydroxyphenylglycol, 3,4-dihydroxymandelic acid, and 3,4-dihydroxyphenylglycol. Norepinephrine metabolites are excreted in urine primarily as the sulfate conjugates and, to a lesser extent, as the glucuronide conjugates. Only small quantities of norepinephrine are excreted unchanged.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1400
Norepinephrine functions as a peripheral vasoconstrictor by acting on alpha-adrenergic receptors. It is also an inotropic stimulator of the heart and dilator of coronary arteries as a result of it's activity at the beta-adrenergic receptors.
The pharmacological actions of norepinephrine and epinephrine have been extensively compared in vivo and in vitro. Both drugs are direct agonists on effector cells, and their actions differ mainly in the ratio of their effectiveness in stimulating alpha and beta2-receptors. They are approximately equipotent in stimulating beta1-receptors. Norepinephrine is a potent alpha agonist and has relatively little action on beta-2 receptors; however, it is somewhat less potent than epinephrine on the alpha receptors of most organs.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 248
During cold exposures there is an immediate release of catecholamines (e.g., norepinephrine, dopamine), which activate the sympathetic nervous system to reduce heat loss via peripheral vasoconstriction and shift substrate utilization toward fatty acid metabolism for heat production.
Mahar H; Patty's Toxicology CD-ROM (2005). NY, NY: John Wiley & Sons; Cold Stress and Strain. Online Posting Date: April 16, 2001.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
19
PharmaCompass offers a list of Noradrenaline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Noradrenaline manufacturer or Noradrenaline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Noradrenaline manufacturer or Noradrenaline supplier.
PharmaCompass also assists you with knowing the Noradrenaline API Price utilized in the formulation of products. Noradrenaline API Price is not always fixed or binding as the Noradrenaline Price is obtained through a variety of data sources. The Noradrenaline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Noradrenaline Hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Noradrenaline Hydrochloride, including repackagers and relabelers. The FDA regulates Noradrenaline Hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Noradrenaline Hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Noradrenaline Hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Noradrenaline Hydrochloride supplier is an individual or a company that provides Noradrenaline Hydrochloride active pharmaceutical ingredient (API) or Noradrenaline Hydrochloride finished formulations upon request. The Noradrenaline Hydrochloride suppliers may include Noradrenaline Hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Noradrenaline Hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Noradrenaline Hydrochloride CEP of the European Pharmacopoeia monograph is often referred to as a Noradrenaline Hydrochloride Certificate of Suitability (COS). The purpose of a Noradrenaline Hydrochloride CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Noradrenaline Hydrochloride EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Noradrenaline Hydrochloride to their clients by showing that a Noradrenaline Hydrochloride CEP has been issued for it. The manufacturer submits a Noradrenaline Hydrochloride CEP (COS) as part of the market authorization procedure, and it takes on the role of a Noradrenaline Hydrochloride CEP holder for the record. Additionally, the data presented in the Noradrenaline Hydrochloride CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Noradrenaline Hydrochloride DMF.
A Noradrenaline Hydrochloride CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Noradrenaline Hydrochloride CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Noradrenaline Hydrochloride suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Noradrenaline Hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Noradrenaline Hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Noradrenaline Hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Noradrenaline Hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Noradrenaline Hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Noradrenaline Hydrochloride suppliers with NDC on PharmaCompass.
Noradrenaline Hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Noradrenaline Hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Noradrenaline Hydrochloride GMP manufacturer or Noradrenaline Hydrochloride GMP API supplier for your needs.
A Noradrenaline Hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Noradrenaline Hydrochloride's compliance with Noradrenaline Hydrochloride specifications and serves as a tool for batch-level quality control.
Noradrenaline Hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Noradrenaline Hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Noradrenaline Hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Noradrenaline Hydrochloride EP), Noradrenaline Hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Noradrenaline Hydrochloride USP).
