
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Australia

0
Listed Dossiers
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
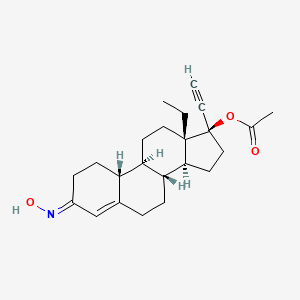

1. Norgestrel Oxime Acetate
1. Dexnorgestrel Acetime
2. 35189-28-7
3. Anti-norgestimate
4. Norgestimate, E-
5. Norgestimato
6. Norgestimatum
7. Norgestimatum [inn-latin]
8. Norgestimato [inn-spanish]
9. Orf-10131
10. Nkx8dn6ty9
11. [(3e,8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-3-hydroxyimino-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl] Acetate
12. (+)-13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one Oxime Acetate (ester)
13. 20799-27-3
14. Chebi:50815
15. Orf 10131
16. Rwj 10131
17. Ncgc00181353-01
18. (17alpha)-17-(acetyloxy)-13-ethyl-18,19-dinorpregn-4-en-20-yn-3-one 3-oxime
19. Dsstox_cid_26922
20. Dsstox_rid_82018
21. Dsstox_gsid_46922
22. 107382-52-5
23. D-13beta-ethyl-17alpha-ethynyl-17beta-acetoxygon-4-en-3-one Oxime
24. Rwj-10131
25. Cas-35189-28-7
26. Norgestimate (usp/inn)
27. Nsc-759159
28. Unii-nkx8dn6ty9
29. Schembl38317
30. Schembl38318
31. Chembl1200934
32. Dtxsid1046922
33. Norgestimate, >=97% (hplc)
34. Norgestimate For System Suitability
35. Tox21_112811
36. Ac-655
37. (3e)-17alpha-ethynyl-3-(hydroxyimino)-18a-homoestr-4-en-17beta-yl Acetate
38. Akos015917555
39. Tox21_112811_1
40. Db00957
41. Ncgc00344561-01
42. As-12305
43. D05209
44. 351n287
45. A822635
46. J-019983
47. 18,19-dinorpregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-, 3-oxime, (3e,17alpha)-
48. (1s,2r,5e,10r,11s,14r,15s)-15-ethyl-14-ethynyl-5-(hydroxyimino)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl Acetate
49. [(3e,8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-3-hydroxyimino-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl] Acetate;norgestimate
50. 18,19-dinorpregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-, 3-oxime, (3e,17.alpha.)-
| Molecular Weight | 369.5 g/mol |
|---|---|
| Molecular Formula | C23H31NO3 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 369.23039385 g/mol |
| Monoisotopic Mass | 369.23039385 g/mol |
| Topological Polar Surface Area | 58.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 744 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Contraceptives, Oral, Synthetic; Norgestrel/analogs & derivatives
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Norgestimate/ethinyl estradiol is indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method of contraception. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
/BOXED WARNING/ WARNINGS: CARDIOVASCULAR RISK ASSOCIATED WITH SMOKING. Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, combination oral contraceptives, including MonoNessa, should not be used by women who are over 35 years of age and smoke.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (Updated: February 2015). Available from, as of April 23, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=704ba4ae-edd0-4298-a399-03a49f8411c7
Oral contraceptives should not be used in women who currently have the following conditions: thrombophlebitis or thromboembolic disorders; a past history of deep vein thrombophlebitis or thromboembolic disorders; cerebral vascular or coronary artery disease (current or past history); valvular heart disease with complications; severe hypertension; diabetes with vascular involvement; headaches with focal neurological symptoms; major surgery with prolonged immobilization; known or suspected carcinoma of the breast or personal history of breast cancer; carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia; undiagnosed abnormal genital bleeding; cholestatic jaundice of pregnancy or jaundice with prior pill use; acute or chronic hepatocellular disease with abnormal liver function; hepatic adenomas or carcinomas; known or suspected pregnancy; hypersensitivity to any component of this product.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity and diabetes.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
Oral contraceptives have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (>35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and nonusers, for both types of strokes, and smoking interacted to increase the risk of stroke. In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension. The relative risk of hemorrhagic stroke is reported to be 1.2 for non-smokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension. The attributable risk is also greater in older women.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
For more Drug Warnings (Complete) data for Norgestimate (44 total), please visit the HSDB record page.
Norgestimate is formulated with [ethinylestradiol] as a combined oral contraceptive. It can also be given with low dose ethinylestradiol for contraception as well as the treatment of moderate acne vulgaris in women 15 years old.
Norgestimate is a progestin that suppresses ovulation for contraception and reduces free testosterone to treat moderate acne vulgaris. The therapeutic index is wide as overdoses are rare. Patients should be counselled regarding the risk of vascular problems, liver disease, hypertension, metabolic effects, headaches, and bleeding irregularities.
Contraceptives, Oral, Synthetic
Oral contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Oral, Synthetic.)
Contraceptive Agents, Hormonal
Contraceptive agents that act on the ENDOCRINE SYSTEM. (See all compounds classified as Contraceptive Agents, Hormonal.)
Absorption
Oral norgestimate has a Tmax of 0.5-2h. On day 21 of cycle 3, 17-desacetylnorgestimate reaches a Cmax of 1.82ng/mL, with a Tmax of 1.5h, and an AUC of 16.1h\*ng/mL. At the same time, norgestrel reaches a Cmax of 2.79ng/mL, with a Tmax of 1.7h, and an AUC of 49.9h\*ng/mL.
Route of Elimination
Norgestimate is 45-49% eliminated in urine and 16-49% eliminated in feces. Unchanged norgestimate is not detected in urine.
Volume of Distribution
Data regarding the volume of distribution of norgestimate are not readily available.
Clearance
Data regarding the clearance of norgestimate is not readily available.
Norgestimate (NGM) and ethinyl estradiol (EE) are rapidly absorbed following oral administration.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
Peak serum concentrations of norelgestromin (NGMN) and ethinyl estradiol (EE) are generally reached by 2 hours after administration of MonoNessa. Accumulation following multiple dosing of the 250 ug Norgestimate (NGM)/ 35 ug dose is approximately 2-fold for NGMN and EE compared with single dose administration. The pharmacokinetics of NGMN is dose proportional following NGM doses of 180 ug to 250 ug. ... Steady-state concentrations of NGMN and NG are achieved by Day 21. Non-linear accumulation (approximately 8 fold) of norgestrel is observed as a result of high affinity binding to SHBG (sex hormone-binding globulin), which limits its biological activity.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
Norelgestromin and norgestrel are highly bound (>97%) to serum proteins. Norelgestromin is bound to albumin and not to SHBG, while norgestrel is bound primarily to SHBG.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
The metabolites of norelgestromin and ethinyl estradiol are eliminated by renal and fecal pathways. Following administration of 14C-norgestimate, 47% (45-49%) and 37% (16-49%) of the administered radioactivity was eliminated in the urine and feces, respectively. Unchanged norgestimate was not detected in the urine.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
Norgestimate is rapidly deacetylated to the active 17-desacetylnorgestimate, which is deoximated to the active norgestrel. 17-desacetylnorgestimate is metabolized to a number of undefined hydroxylated metabolites, mainly by CYP3A4 and to a lesser extend by CYP2B6 and CYP2C9. Norgestrel is O-glucuronidated by UGT1A1 or oxidized to a number of undefined hydroxylated metabolites by CYP3A4.
Norgestimate is rapidly and completely metabolized by first pass (intestinal and/or hepatic) mechanisms to norelgestromin (NGMN) and norgestrel (NG), which are the major active metabolites of norgestimate.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
Norgestimate is extensively metabolized by first-pass mechanisms in the gastrointestinal tract and/or liver. Norgestimate's primary active metabolite is norelgestromin. Subsequent hepatic metabolism of norelgestromin occurs and metabolites include norgestrel, which is also active, and various hydroxylated and conjugated metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
In addition to 17-deacetyl norgestimate, a number of metabolites of norgestimate have been identified in human urine following administration of radiolabeled norgestimate. These include 18,19-Dinor-17-pregn-4-en-20-yn-3-one,17-hydroxy-13-ethyl,(17alpha)-(-); 18,19-Dinor-5beta-17-pregnan-20-yn,3alpha,17beta-dihydroxy-13-ethyl,(17alpha), various hydroxylated metabolites and conjugates of these metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
There is limited information on the metabolism of levonorgestrel, norethindrone and structurally related contraceptive steroids. Both levonorgestrel and norethindrone undergo extensive reduction of the alpha, beta-unsaturated ketone in ring A. Levonorgestrel also undergoes hydroxylation at carbons 2 and 16. The metabolites of both compounds circulate predominantly as sulfates. In urine, levonorgestrel metabolites are found primarily in the glucuronide form, whereas norethindrone metabolites are present in approximately equal amounts as sulfates and glucuronides. Of the progestogens structurally related to norethindrone, norethindrone acetate, ethynodiol diacetate, norethindrone enanthate, and perhaps lynestrenol, undergo rapid hydrolysis and are converted to the parent compound and its metabolites. There is no convincing evidence that norethynodrel is converted to norethindrone. Of the progestogens structurally related to levonorgestrel, it appears that neither desogestrel nor gestodene are transformed to the parent compound. However, there is evidence that norgestimate can be, at least partly, converted to levonorgestrel. ...
PMID:2143719 Stanczyk FZ, Roy S; Contraception 42 (1): 67-96 (1990)
Norgestimate is rapidly deacetylated. The active metabolites of norgestimate, 17-desacetyl norgestimate, has a half life of 12-30h, while norgestrel has a half life of 36.410.2h.
Progesterone analogs like norgestimate decrease the frequency of gonadotropin releasing hormone pulses from the hypothalamus, decreasing follicle stimulating hormone and luteinizing hormone. These actions prevent ovulation. Norgestimate suppresses the hypothalamo-pituitary-axis, reducing androgen synthesis. It also induces production of sex hormone binding globulin, which decreases free testosterone. These actions together result in less testosterone being available to stimulate sebaceous glands, resulting in effective treatment of some forms of acne.
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
Receptor binding studies, as well as studies in animals and humans, have shown that norgestimate and 17-deacetyl norgestimate, the major serum metabolite, combine high progestational activity with minimal intrinsic androgenicity. Norgestimate, in combination with ethinyl estradiol, does not counteract the estrogen-induced increases in sex hormone binding globulin (SHBG), resulting in lower serum testosterone.
US Natl Inst Health; DailyMed. Current Medication Information for MONONESSA (norgestimate and ethinyl estradiol) kit (December 2010). Available from, as of March 30, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=33976
Progestins enter target cells by passive diffusion and bind to cytosolic (soluble) receptors that are loosely bound in the nucleus. The steroid receptor complex initiates transcription, resulting in an increase in protein synthesis. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
Progestins are capable of affecting serum concentrations of other hormones, particularly estrogen. Estrogenic effects are modified by the progestins, either by reducing the availability or stability of the hormone receptor complex or by turning off specific hormone-responsive genes by direct interaction with the progestin receptor in the nucleus. In addition, estrogen priming is necessary to increase progestin effects by upregulating the number of progestin receptors and/or increasing progesterone production, causing a negative feedback mechanism that inhibits estrogen receptors. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
41
PharmaCompass offers a list of Norgestimate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Norgestimate manufacturer or Norgestimate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Norgestimate manufacturer or Norgestimate supplier.
PharmaCompass also assists you with knowing the Norgestimate API Price utilized in the formulation of products. Norgestimate API Price is not always fixed or binding as the Norgestimate Price is obtained through a variety of data sources. The Norgestimate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Norgestimate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Norgestimate, including repackagers and relabelers. The FDA regulates Norgestimate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Norgestimate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Norgestimate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Norgestimate supplier is an individual or a company that provides Norgestimate active pharmaceutical ingredient (API) or Norgestimate finished formulations upon request. The Norgestimate suppliers may include Norgestimate API manufacturers, exporters, distributors and traders.
click here to find a list of Norgestimate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Norgestimate DMF (Drug Master File) is a document detailing the whole manufacturing process of Norgestimate active pharmaceutical ingredient (API) in detail. Different forms of Norgestimate DMFs exist exist since differing nations have different regulations, such as Norgestimate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Norgestimate DMF submitted to regulatory agencies in the US is known as a USDMF. Norgestimate USDMF includes data on Norgestimate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Norgestimate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Norgestimate suppliers with USDMF on PharmaCompass.
A Norgestimate CEP of the European Pharmacopoeia monograph is often referred to as a Norgestimate Certificate of Suitability (COS). The purpose of a Norgestimate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Norgestimate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Norgestimate to their clients by showing that a Norgestimate CEP has been issued for it. The manufacturer submits a Norgestimate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Norgestimate CEP holder for the record. Additionally, the data presented in the Norgestimate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Norgestimate DMF.
A Norgestimate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Norgestimate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Norgestimate suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Norgestimate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Norgestimate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Norgestimate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Norgestimate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Norgestimate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Norgestimate suppliers with NDC on PharmaCompass.
Norgestimate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Norgestimate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Norgestimate GMP manufacturer or Norgestimate GMP API supplier for your needs.
A Norgestimate CoA (Certificate of Analysis) is a formal document that attests to Norgestimate's compliance with Norgestimate specifications and serves as a tool for batch-level quality control.
Norgestimate CoA mostly includes findings from lab analyses of a specific batch. For each Norgestimate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Norgestimate may be tested according to a variety of international standards, such as European Pharmacopoeia (Norgestimate EP), Norgestimate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Norgestimate USP).

