
Synopsis
Synopsis
0
KDMF
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
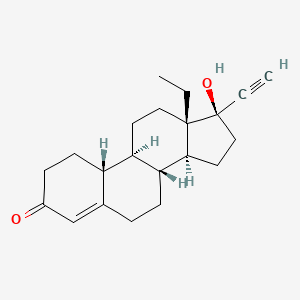

1. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17alpha)-(-)-
2. Capronor
3. Cerazet
4. D Norgestrel
5. D-norgestrel
6. Duofem
7. L Norgestrel
8. L-norgestrel
9. Microlut
10. Microval
11. Mirena
12. Norgeston
13. Norlevo
14. Norplant
15. Norplant 2
16. Norplant-2
17. Norplant2
18. Plan B
19. Vikela
1. Norgestrel
2. 797-63-7
3. D-norgestrel
4. Mirena
5. (-)-norgestrel
6. Levonova
7. Microval
8. Postinor
9. Plan B
10. Norplant
11. Jadelle
12. Follistrel
13. 18-methylnorethisterone
14. Levonorgestrelum
15. Ovrette
16. 6533-00-2
17. D(-)-norgestrel
18. Levonelle
19. D-(-)-norgestrel
20. Neogest
21. Levonorgestrelum [inn-latin]
22. Liletta
23. Fallback Solo
24. Monovar
25. Wy-5104
26. Dl-norgestrel
27. Norgestrel-(-)-d
28. 17-ethynyl-18-methyl-19-nortestosterone
29. 17alpha-ethynyl-18-homo-19-nortestosterone
30. 18-methyl-17-alpha-ethynyl-19-nortestosterone
31. 13-ethyl-17-alpha-ethynylgon-4-en-17-beta-ol-3-one
32. 17alpha-ethynyl-17-hydroxy-18-methylestr-4-en-3-one
33. Norgestrelum
34. 17alpha-ethynyl-13beta-ethyl-3-oxo-4-estren-17beta-ol
35. 13-ethyl-17-alpha-ethynyl-17-beta-hydroxy-4-gonen-3-one
36. Bay86-5028
37. Norgestrel, (-)-
38. Norgestrel (-)-form
39. Norplant 2
40. Microlution
41. Ovranette
42. Triagynon
43. Triciclor
44. Microgyn
45. Microlut
46. 5w7sia7yzw
47. Nordet
48. Trigoa
49. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one
50. Wy 3707
51. Wy-3707
52. 17-alpha-ethinyl-13-beta-ethyl-17-beta-hydroxy-4-estren-3-one
53. Nsc-744007
54. Microgynon Cd
55. Microgest Ed
56. Norplant Ii
57. (-)-13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one
58. Logynon Ed
59. Ovral-lo
60. Levlen Ed
61. Microgynon 21
62. Microgynon 28
63. Trinordiol 21
64. Trinordiol 28
65. Mls000069491
66. Chebi:6443
67. Minivlar 30
68. Monofeme 28
69. Neogynon 21
70. Nordette 21
71. Nordette 28
72. Stediril 30
73. Trifeme 28
74. Preven
75. Levora-21
76. Levora-28
77. Microgynon 30 Ed
78. Tri-levlen 21
79. 17-alpha-ethynyl-13-ethyl-19-nortestosterone
80. Rigevidon 21+7
81. Fh 122-a
82. Norlevo
83. 13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one
84. Sh 850
85. Triquilar Ed
86. Ld Norgestrel [french]
87. Triphasil 21
88. Triphasil 28
89. 3j8q1747z2
90. Sh 70850
91. Ncgc00159349-02
92. Norgestrelum [inn-latin]
93. 13-beta-ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one
94. Smr000059117
95. Levonorgestrel Implants
96. E-gen-c
97. Norgestrel [progestins]
98. Ovoplex 30-150
99. Dsstox_cid_16496
100. Dsstox_rid_79283
101. Dsstox_gsid_36496
102. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3h-cyclopenta[a]phenanthren-3-one
103. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3(2h)-one
104. Microluton
105. Levogel
106. Levosert
107. Skyla
108. 13-ethyl-17alpha-hydroxy-18,19-dinorpregn-4-en-20-yn-3-one
109. Next Choice
110. Norplant System In Plastic Container
111. Plan B One Step
112. Plan B One-step
113. Norplant (tn)
114. Rel-(8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3(2h)-one
115. Ovrette (tn)
116. Mirena (tn)
117. Ccris 6525
118. Ccris 9033
119. Levonorgestrel (levonelle)
120. Hsdb 3595
121. Hsdb 6483
122. Lng-ius
123. 17alpha-ethynyl-13-ethyl-19-nortestosterone
124. 13beta-ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one
125. Einecs 212-349-8
126. Einecs 229-433-5
127. Unii-5w7sia7yzw
128. Brn 2391114
129. Fh-122a
130. 13-ethyl-17alpha-ethynylgon-4-en-17beta-ol-3-one
131. Kyleena
132. Dl-13-beta-ethyl-17-alpha-ethynyl-19-nortestosterone
133. Sh-850
134. 19-nortestosterone, 17-ethynyl-18-methyl-
135. Levonorgestrel (jan/usp/inn)
136. Unii-3j8q1747z2
137. 1lhv
138. Nsc-757251
139. Oral Levonorgestrel
140. Cas-797-63-7
141. Lng
142. Sh-70850
143. Prestwick_109
144. (+-)-norgestrel
145. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17alpha)-(+-)-
146. (-)-levonorgestrel
147. Levonorgestrel [usan:usp:inn:ban]
148. Mfcd00199013
149. Levonorgestrel Implant
150. Skyla (tn)
151. Norgestrel [usan:usp:inn:ban:jan]
152. Bay 86-5028
153. Norgestrel [mi]
154. Opera_id_552
155. Levonorgestrel(levonelle)
156. Norgestrel [inn]
157. Norgestrel [jan]
158. Dl-13-beta-ethyl-17-alpha-ethynyl-17-beta-hydroxygon-4-en-3-one
159. Prestwick0_000773
160. Prestwick1_000773
161. Prestwick2_000773
162. Prestwick3_000773
163. Norgestrel [hsdb]
164. Norgestrel [usan]
165. Intrauterine Levonorgestrel
166. 17-beta-hydroxy-18-methyl-19-nor-17-alpha-pregn-4-en-20-yn-3-one
167. Norgestrel [vandf]
168. (+-)-13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one
169. (-)-norgestrel, 98%
170. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17alpha)-(-)-
171. Norgestrel [mart.]
172. Chembl1389
173. Levonorgestrel [inn]
174. Levonorgestrel [jan]
175. Norgestrel [usp-rs]
176. Norgestrel [who-dd]
177. Bidd:pxr0194
178. Schembl27597
179. Bspbio_000846
180. Levonorgestrel [hsdb]
181. Levonorgestrel [usan]
182. Mls000759484
183. Mls001074069
184. Mls001423967
185. Levonorgestrel [vandf]
186. Spbio_002785
187. Levonorgestrel [mart.]
188. 13-ethyl-17alpha-ethynyl-17-hydroxygon-4-en-3-one
189. Bpbio1_000932
190. Gtpl2881
191. Norgestrel (jp17/usp/inn)
192. Levonorgestrel [usp-rs]
193. Levonorgestrel [who-dd]
194. Levonorgestrel [who-ip]
195. Dtxsid3036496
196. Dtxsid3047477
197. Norgestrel [orange Book]
198. Ovral Component Norgestrel
199. 17alpha-ethynyl-17beta-hydroxy-18a-homoestr-4-en-3-one
200. Norgestrel [ep Monograph]
201. Implant With Levonorgestrel
202. Hms1570k08
203. Hms2051m08
204. Hms2090o06
205. Hms2097k08
206. Hms2232h06
207. Hms2232k12
208. Hms3649j10
209. Hms3714k08
210. Hms3886k18
211. Norgestrel [usp Monograph]
212. (1s,2r,10r,11s,14r,15s)-15-ethyl-14-ethynyl-14-hydroxytetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one
213. Amy40476
214. Cryselle Component Norgestrel
215. Hy-b0257
216. Norgestrel (-)-form [mi]
217. Zinc3814395
218. D-(-)-norgestrel (levonorgestrel)
219. Levonorgestrel [orange Book]
220. Norgestrel Component Of Ovral
221. Tox21_111593
222. Tox21_202872
223. Tox21_303658
224. Bdbm50410522
225. Levonorgestrel [ep Monograph]
226. Levonorgestrel [usp Impurity]
227. Lmst02030119
228. Nsc744007
229. S1727
230. S5709
231. Alesse Component Levonorgestrel
232. Aviane Component Levonorgestrel
233. D(-)-norgestrel, Analytical Standard
234. Levonorgestrel [usp Monograph]
235. Levora Component Levonorgestrel
236. Lybrel Component Levonorgestrel
237. Portia Component Levonorgestrel
238. Preven Component Levonorgestrel
239. Twirla Component Levonorgestrel
240. Vienva Component Levonorgestrel
241. 18,19-dinor-17-alpha-pregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-
242. Akos015894913
243. Kurvelo Component Levonorgestrel
244. Kyleena Component Levonorgestrel
245. Lessina Component Levonorgestrel
246. Levlite Component Levonorgestrel
247. Myzilra Component Levonorgestrel
248. Plastic Iud With Levonorgestrel
249. Tox21_111593_1
250. Trivora Component Levonorgestrel
251. Bcp9000852
252. Ccg-100853
253. Db00367
254. Levonorgestrel For System Suitability 1
255. Levonorgestrel For System Suitability 2
256. Levonorgestrelum [who-ip Latin]
257. Nc00103
258. Norgestrel Component Of Cryselle
259. Nsc 744007
260. Nsc 757251
261. Nsc 759653
262. 18,19-dinor-17-alpha-pregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (+-)-
263. Altavera Component Levonorgestrel
264. Elifemme Component Levonorgestrel
265. Enpresse Component Levonorgestrel
266. Levonest Component Levonorgestrel
267. Marlissa Component Levonorgestrel
268. Nordette Component Levonorgestrel
269. Orsythia Component Levonorgestrel
270. Quasense Component Levonorgestrel
271. Setlakin Component Levonorgestrel
272. [18,(17.alpha.)-(-)-]
273. Afirmelle Component Levonorgestrel
274. Introvale Component Levonorgestrel
275. Levonorgestrel Component Of Alesse
276. Levonorgestrel Component Of Lybrel
277. Levonorgestrel Component Of Preven
278. Levonorgestrel Component Of Twirla
279. Ncgc00159349-03
280. Ncgc00159349-05
281. Ncgc00257283-01
282. Ncgc00260418-01
283. Quartette Component Levonorgestrel
284. Seasonale Component Levonorgestrel
285. Triphasil Component Levonorgestrel
286. Levonorgestrel Component Of Kyleena
287. Levonorgestrel Component Of Levlite
288. Norgestrel 100 Microg/ml In Acetonitrile
289. Seasonique Component Levonorgestrel
290. Smr000653526
291. Climara Pro Component Levonorgestrel
292. Levonorgestrel Component Of Altavera
293. Levonorgestrel Component Of Levonest
294. Levonorgestrel Component Of Quasense
295. Levonorgestrel Component Of Setlakin
296. Levonorgestrel Component Of Introvale
297. Levonorgestrel Component Of Seasonale
298. Loseasonique Component Levonorgestrel
299. Levonorgestrel Component Of Seasonique
300. N0889
301. Norgestimate Impurity B [ep Impurity]
302. Levonorgestrel 100 Microg/ml In Acetonitrile
303. Levonorgestrel Component Of Climara Pro
304. C08149
305. C08153
306. D00950
307. D00954
308. L-4455
309. Levonorgestrel Component Of Loseasonique
310. Opcicon One-step Component Levonorgestrel
311. 797l637
312. Q416950
313. Sr-01000759218
314. Sr-01000946725
315. (-)-17alpha-ethynyl-18-methyl-19-nortestosterone
316. Levonorgestrel Component Of Opcicon One-step
317. Sr-01000759218-5
318. Sr-01000946725-1
319. Brd-k35189033-001-03-0
320. 13-ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one
321. Z1551429747
322. 13beta-ethyl-17alpha-ethynyl-17-hydroxy-gon-4-en-3-one
323. (17?)-13-ethyl-17-hydroxy-18,19-dinorpregn-4-en-yn-3-one
324. 13beta-ethyl-17alpha-ethynyl-17beta-hydroxy-gon-4-en-3-one
325. Levonorgestrel, British Pharmacopoeia (bp) Reference Standard
326. Levonorgestrel, European Pharmacopoeia (ep) Reference Standard
327. Levonorgestrel, United States Pharmacopeia (usp) Reference Standard
328. (-)-18,19-dinor-13beta-ethyl-17beta-hydroxy-4-pregnen-20-yn-3-one
329. [(-)-13-ethyl-17-hydroxy-18,19-dinor-17a-pregn-4-en-20-yn-3-one
330. (+/-)-13-ethyl-17-hydroxy-18,19-dinor-17.alpha.-pregn-4-en-20-yn-3-one
331. (-)-13-ethyl-17-hydroxy-18,19-dinor-17.alpha.-pregn-4-en-20-yn-3-one
332. [18,19-dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-,(17..)-(-)-]
333. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17.alpha.)-(+/-)-
334. 18,19-dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17.alpha.)-(-)-
335. Levonorgestrel For System Suitability 2, European Pharmacopoeia (ep) Reference Standard
336. Levonorgestrel, Pharmaceutical Secondary Standard; Certified Reference Material
337. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy- 1,2,6,7,8,9,10,11,12,13,14,15,16, 17- Tetradecahydrocyclopenta[a] Phenanthren-3-one
338. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3h-cyclopenta[a]phenanthren-3-one (non-preferred Name)
| Molecular Weight | 312.4 g/mol |
|---|---|
| Molecular Formula | C21H28O2 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 312.208930132 g/mol |
| Monoisotopic Mass | 312.208930132 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 609 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 14 | |
|---|---|
| Drug Name | Fallback solo |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1.5mg |
| Market Status | Over the Counter |
| Company | Lupin |
| 2 of 14 | |
|---|---|
| Drug Name | Levonorgestrel |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Emergency contraceptive tablet. Each Next ChoiceTM tablet contains 0.75 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17)-(-)-], a totally synthetic progestogen. The inactive ingr... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.75mg; 1.5mg |
| Market Status | Over the Counter; Prescription |
| Company | Lupin; Novel Labs; Perrigo R And D; Watson Labs |
| 3 of 14 | |
|---|---|
| Drug Name | Mirena |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Mirena is intended to provide an initial release rate of 20 mcg/day of levonorgestrelLevonorgestrel USP, (-)-13-Ethyl-17-hydroxy-18,19-dinor-17-pregn-4-en-20-yn-3-one, the active ingredient in Mirena, has a molecular weight of 312.4, a molecular fo... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Intrauterine device |
| Route | Intrauterine |
| Strength | 52mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 4 of 14 | |
|---|---|
| Drug Name | Plan b |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Each Plan B tablet contains 0.75 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17)-(-)-], a totally synthetic progestogen. The inactive ingredients present are colloidal silicon d... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.75mg |
| Market Status | Over the Counter; Prescription |
| Company | Teva Branded Pharm |
| 5 of 14 | |
|---|---|
| Drug Name | Plan b one-step |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | The Plan B One-Step tablet contains 1.5 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17 )-(-)-], a totally synthetic progestogen. The inactive ingredients are colloidal silicon d... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1.5mg |
| Market Status | Over the Counter |
| Company | Teva Branded Pharm |
| 6 of 14 | |
|---|---|
| Drug Name | Prefest |
| PubMed Health | Estradiol |
| Drug Classes | Endocrine-Metabolic Agent, Female Reproductive Agent, Hormonal Contraceptive, Musculoskeletal Agent |
| Active Ingredient | norgestimate; Estradiol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg,1mg; n/a,0.09mg |
| Market Status | Prescription |
| Company | Teva Womens |
| 7 of 14 | |
|---|---|
| Drug Name | Skyla |
| Drug Label | Skyla (LNG-releasing intrauterine system) contains 13.5 mg of LNG, a progestin, and is intended to provide an initial release rate of approximately14 mcg/day of LNG after 24 days.Levonorgestrel USP, (-)-13-Ethyl-17-hydroxy-18,19-dinor-17-pregn-4-en... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Intrauterine device |
| Route | Intrauterine |
| Strength | 13.5mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 8 of 14 | |
|---|---|
| Drug Name | Fallback solo |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1.5mg |
| Market Status | Over the Counter |
| Company | Lupin |
| 9 of 14 | |
|---|---|
| Drug Name | Levonorgestrel |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Emergency contraceptive tablet. Each Next ChoiceTM tablet contains 0.75 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17)-(-)-], a totally synthetic progestogen. The inactive ingr... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.75mg; 1.5mg |
| Market Status | Over the Counter; Prescription |
| Company | Lupin; Novel Labs; Perrigo R And D; Watson Labs |
| 10 of 14 | |
|---|---|
| Drug Name | Mirena |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Mirena is intended to provide an initial release rate of 20 mcg/day of levonorgestrelLevonorgestrel USP, (-)-13-Ethyl-17-hydroxy-18,19-dinor-17-pregn-4-en-20-yn-3-one, the active ingredient in Mirena, has a molecular weight of 312.4, a molecular fo... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Intrauterine device |
| Route | Intrauterine |
| Strength | 52mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 11 of 14 | |
|---|---|
| Drug Name | Plan b |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | Each Plan B tablet contains 0.75 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17)-(-)-], a totally synthetic progestogen. The inactive ingredients present are colloidal silicon d... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.75mg |
| Market Status | Over the Counter; Prescription |
| Company | Teva Branded Pharm |
| 12 of 14 | |
|---|---|
| Drug Name | Plan b one-step |
| PubMed Health | Levonorgestrel |
| Drug Classes | Contraceptive, Contraceptive, Local, Contraceptive, Progestin, Endocrine-Metabolic Agent |
| Drug Label | The Plan B One-Step tablet contains 1.5 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17 )-(-)-], a totally synthetic progestogen. The inactive ingredients are colloidal silicon d... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1.5mg |
| Market Status | Over the Counter |
| Company | Teva Branded Pharm |
| 13 of 14 | |
|---|---|
| Drug Name | Prefest |
| PubMed Health | Estradiol |
| Drug Classes | Endocrine-Metabolic Agent, Female Reproductive Agent, Hormonal Contraceptive, Musculoskeletal Agent |
| Active Ingredient | norgestimate; Estradiol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg,1mg; n/a,0.09mg |
| Market Status | Prescription |
| Company | Teva Womens |
| 14 of 14 | |
|---|---|
| Drug Name | Skyla |
| Drug Label | Skyla (LNG-releasing intrauterine system) contains 13.5 mg of LNG, a progestin, and is intended to provide an initial release rate of approximately14 mcg/day of LNG after 24 days.Levonorgestrel USP, (-)-13-Ethyl-17-hydroxy-18,19-dinor-17-pregn-4-en... |
| Active Ingredient | Levonorgestrel |
| Dosage Form | Intrauterine device |
| Route | Intrauterine |
| Strength | 13.5mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
Contraceptives, Oral, Synthetic; Progestational Hormones, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Low-ogestrel (norgestrel and ethinyl estradiol tablets) is indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
/Cyclo-Progynova is indicated as/ hormone replacement therapy (HRT) for estrogen deficiency symptoms in perimenopausal and postmenopausal women.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyclo-Progynova (Last updated March 2011). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/9159/SPC/Cyclo-Progynova+2mg/
/Cyclo-Progynova is indicated for/ prevention of osteoporosis in postmenopausal women at high risk of future fractures who are intolerant of, or contraindicated for, other medicinal products approved for the prevention of osteoporosis.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyclo-Progynova (Last updated March 2011). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/9159/SPC/Cyclo-Progynova+2mg/
Norgestrel ... /is/ indicated for the prevention of pregnancy. Progestin-only oral contraceptives are also called minipills and progestin-only oral pills (POPs). /Former/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2567
Contraceptive Agents, Female; Contraceptives, Oral, Synthetic; Progestational Hormones, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Aviane is indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method of contraception. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for AVIANE (levonorgestrel and ethinyl estradiol) kit (September 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14957
Next Choice is a progestin-only emergency contraceptive indicated for prevention of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. To obtain optimal efficacy, the first tablet should be taken as soon as possible within 72 hours of intercourse. The second tablet should be taken 12 hours later. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for NEXT CHOICE (levonorgestrel) tablet (December 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14292
Mirena is indicated for the treatment of heavy menstrual bleeding in women who choose to use intrauterine contraception as their method of contraception. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for MIRENA (levonorgestrel) intrauterine device (May 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11844
For more Therapeutic Uses (Complete) data for LEVONORGESTREL (7 total), please visit the HSDB record page.
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, hypercholesterolemia, obesity and diabetes.
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
Oral contraceptives should not be used in women who have the following conditions: thrombophlebitis or thromboembolic disorders; a past history of deep vein thrombophlebitis or thromboembolic disorders; cerebral vascular or coronary artery disease; Known or suspected carcinoma of the breast; carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia; undiagnosed abnormal genital bleeding; cholestatic jaundice of pregnancy or jaundice with prior pill use; hepatic adenomas, carcinomas or benign liver tumors; known or suspected pregnancy
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
The most frequent adverse effect of oral contraceptives is nausea. In addition, nausea has been reported in women using vaginal or transdermal estrogen-progestin contraceptives. The principal risk associated with currently recommended high-dose, postcoital estrogen-progestin combination regimens appears to be moderate to severe adverse GI effects including severe vomiting and nausea, which occur in 12-22 and 30-66%, respectively, of women receiving the short-course regimens and may limit compliance with, and effectiveness of, the regimens. In 2 prospective, randomized studies, nausea and vomiting were less common with a high-dose postcoital progestin-only regimen (0.75 mg levonorgestrel every 12 hours for 2 doses) than with a high-dose estrogen-progestin regimen (100 mcg ethinyl estradiol and 0.5 mg levonorgestrel every 12 hours for 2 doses). Other adverse GI effects include vomiting, abdominal cramps, abdominal pain, bloating, diarrhea, and constipation. Gingivitis and dry socket have also been reported. Changes in appetite and changes in weight also may occur. /Estrogen-Progestin Combination/
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3109
For more Drug Warnings (Complete) data for NORGESTREL (52 total), please visit the HSDB record page.
/BOXED WARNING/ WARNINGS: Estrogens and progestins should not be used for the prevention of cardiovascular disease or dementia. The Women's Health Initiative (WHI) study reported increased risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and deep vein thrombosis in postmenopausal women (50 to 79 years of age) during 5 years of treatment with oral conjugated estrogens (CE 0.625 mg) combined with medroxyprogesterone acetate (MPA 2.5 mg) relative to placebo. The WHI study reported increased risks of stroke and deep vein thrombosis in postmenopausal women (50 to 79 years of age) during 6.8 years of treatment with oral conjugated estrogens (CE 0.625 mg) relative to placebo. The Women's Health Initiative Memory Study (WHIMS), a substudy of WHI, reported increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with CE 0.625 mg combined with MPA 2.5 mg and during 5.2 years of treatment with CE 0.625 mg alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. Other doses of oral conjugated estrogens with medroxyprogesterone acetate, and other combinations and dosage forms of estrogens and progestins were not studied in the WHI clinical trials and, in the absence of comparable data, these risks should be assumed to be similar. Because of these risks, estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
US Natl Inst Health; DailyMed. Current Medication Information for CLIMARA PRO (estradiol and levonorgestrel) patch (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=34043
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with the extent of smoking (in epidemiologic studies, 15 or more cigarettes per day was associated with a significantly increased risk) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
US Natl Inst Health; DailyMed. Current Medication Information for MIRENA (levonorgestrel) intrauterine device (May 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=11844
Use of oral contraceptives is associated with an increased risk of several serious conditions including thromboembolism, stroke, myocardial infarction, liver tumor, gallbladder disease, visual disturbances, fetal abnormalities, and hypertension. Cigarette smoking increases the risk of serious adverse cardiovascular effects during oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes daily) and is markedly greater in women older than 35 years of age. Women who are receiving estrogen-progestin contraceptives should be strongly advised not to smoke. Women older than 35 years of age who smoke, and women with ischemic heart disease or a history of this disease, should not use estrogen-progestin contraceptives. Estrogen-progestin contraceptives should be used with caution in women with cardiovascular disease risk factors.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3112
In general, no adverse effects of progestin-only pills have been found on breastfeeding performance or on the health, growth or development of the infant. However, isolated post-marketing cases of decreased milk production have been reported.
US Natl Inst Health; DailyMed. Current Medication Information for NEXT CHOICE (levonorgestrel) tablet (December 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14292
For more Drug Warnings (Complete) data for LEVONORGESTREL (52 total), please visit the HSDB record page.
**Emergency contraception** Levonorgestrel, in the single-agent emergency contraceptive form, is indicated for the prevention of pregnancy after the confirmed or suspected failure of contraception methods or following unprotected intercourse. It is distributed by prescription for patients under 17, and over the counter for those above this age. This levonorgestrel-only form of contraception is not indicated for regular contraception and must be taken as soon as possible within 72 hours after intercourse. It has shown a lower efficacy when it is used off label within 96 hours. **Long-term contraception or nonemergency contraception** In addition to the above indication in emergency contraception, levonorgestrel is combined with other contraceptives in contraceptive formulations designed for regular use, for example with ethinyl estradiol. It is used in various hormone-releasing intrauterine devices for long-term contraception ranging for a duration of 3-5 years. Product labeling for Mirena specifically mentions that it is recommended in women who have had at least 1 child. A subdermal implant is also available for the prevention of pregnancy for up to 5 years. **Hormone therapy and off-label uses** Levonorgestrel is prescribed in combination with estradiol as hormone therapy during menopause to manage vasomotor symptoms and to prevent osteoporosis.Off-label, levonorgestrel may be used to treat menorrhagia, endometrial hyperplasia, and endometriosis.
Prevention of pregnancy
Contraception
Levonorgestrel prevents pregnancy by interfering with ovulation, fertilization, and implantation. The levonorgestrel-only containing emergency contraceptive tablet is 89% effective if it is used according to prescribing information within 72 hours after intercourse. The intrauterine and implantable devices releasing levonorgestrel are more than 99% in preventing pregnancy. Levonorgestrel utilized as a component of hormonal therapy helps to prevent endometrial carcinoma associated with unopposed estrogen administration.
Contraceptive Agents, Female
Chemical substances or agents with contraceptive activity in females. Use for female contraceptive agents in general or for which there is no specific heading. (See all compounds classified as Contraceptive Agents, Female.)
Contraceptive Agents, Hormonal
Contraceptive agents that act on the ENDOCRINE SYSTEM. (See all compounds classified as Contraceptive Agents, Hormonal.)
Contraceptives, Oral, Synthetic
Oral contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Oral, Synthetic.)
G03AD01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G03FB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03A - Hormonal contraceptives for systemic use
G03AC - Progestogens
G03AC03 - Levonorgestrel
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03A - Hormonal contraceptives for systemic use
G03AD - Emergency contraceptives
G03AD01 - Levonorgestrel
Absorption
Orally administered levonorgestrel is absorbed in the gastrointestinal tract while levonorgestrel administered through an IUD device is absorbed in the endometrium. Levonorgestrel is absorbed immediately in the interstitial fluids when it is inserted as a subdermal implant. After insertion of the subdermal implant, the Cmax of levonorgestrel is attained within 2-3 days.The Cmax following one dose of 0.75 mg of oral levonorgestrel is reached within the hour after administration, according to one reference. In a pharmacokinetic study of 1.5 mg of levonorgestrel in women with a normal BMI and those considered to be obese (BMI>30), mean Cmax was found to be 16.2 ng/mL and 10.5 ng/mL respectively. Tmax was found to be 2 hours for those with normal BMI and 2.5 hours for patients with increased BMI. The bioavailability of levonorgestrel approaches 100%. Mean AUC has been shown to be higher in patients with a normal BMI, measuring at 360.1 h ng/mL versus a range of 197.28 to 208.1 h ng/mL in an obese group of patients. Obesity may contribute to decreased efficacy of levonorgestrel in contraception.
Route of Elimination
Approximately 45% of an oral levonorgestrel dose and its conjugated or sulfate metabolites are found to be excreted in the urine. Approximately 32% of an orally ingested dose is found excreted in feces, primarily in the form of glucuronide conjugates of levonorgestrel.
Volume of Distribution
One pharmacokinetic study determined a mean steady-state volume of distribution of 1.5 mg of levonorgestrel to be 162.2 L in those with normal BMI and in the range of 404.7 L to 466.4 L in obese patients with a body mass index of at least 30. Mean volume of distribution in 16 patients receiving 0.75 mg of levonorgestrel in another pharmacokinetic study was 260 L. The Plan B one-step FDA label reports an apparent volume of distribution of 1.8 L/kg.
Clearance
Clearance was found to 4.8 L/h in healthy female volunteers with a normal BMI, and 7.70-8.51 L/h in obese patients after a single 1.5 mg dose. After a 0.75 mg dose of levonorgestrel in 16 patients in another pharmacokinetic study, mean clearance was calculated at 7.06 L/h. Following levonorgestrel implant removal, the serum concentration falls below 100 pg/mL within the first 96 hours and further falls below the sensitivity of detection within the range of 5 days to 2 weeks.
Norgestrel is absorbed from the gastrointestinal tract, metabolised by the liver and excreted in the urine and faeces as glucuronide and sulphate conjugates.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyclo-Progynova (Last updated March 2011). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/9159/SPC/Cyclo-Progynova+2mg/
(14)C-Norgestrel was administered to seven human subjects and 43% of dose was excreted in the urine within 5 days; the biological half-life of the radioactivity was 24 hr. Enzymic hydrolysis released only 32% of the urinary radioactivity and a further 25% was excreted as sulphate conjugates. The metabolites excreted in the urine were much less polar than those following the administration of the related compounds, norethisterone or lynestrenol. The 3alphaOH,5beta and 3betaOH,5beta isomers of the tetrahydronorgestrel (13beta-ethyl-17alpha-ethynyl-5 beta-gonane-3alpha,17beta-diol) were isolated from urine and identified by mass spectrometry and thin-layer and gas-liquid chromatography. Plasma radioactivity decreased more rapidly than after the administration of norethisterone and lynestrenol. About 2% of the administered dose was converted to acidic compounds. There was no apparent difference in the rate of excretion of radioactivity or in the metabolites after either oral or intravenous administration of norgestrel.
Littleton P et al; Journal of Endocrinology 42: 591-598 (1968)
The binding of different synthetic steroids, used in hormonal contraception, to Sex Hormone Binding Globulin (SHBG) was studied by measuring their ability to displace tritiated testosterone from SHBG in a competitive protein binding system. Only 19-nortestosterone derivates had any significant ability to displace testosterone from SHBG, d-norgestrel (d-Ng) being the strongest displacer. Increasing the SHBG levels in women with previous constant plasma d-Ng levels increased these levels two- to sixfold. It is concluded that SHBG is the main carrier protein for d-Ng. The strong testosterone displacing activity of d-Ng might also explain androgenic side effects observed with d-Ng containig oral contraceptives.
PMID:133117 Victor A et al; J Clin Endocrinol Metab 43 (1): 244-7 (1976)
The apparent volume of distribution of levonorgestrel is reported to be approximately 1.8 L/kg. It is about 97.5 to 99% protein-bound, principally to sex hormone binding globulin (SHBG) and, to a lesser extent, serum albumin.
US Natl Inst Health; DailyMed. Current Medication Information for NEXT CHOICE (levonorgestrel) tablet (December 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14292
No specific investigation of the absolute bioavailability of Aviane (levonorgestrel and ethinyl estradiol tablets, USP) in humans has been conducted. However, literature indicates that levonorgestrel is rapidly and completely absorbed after oral administration (bioavailability about 100%) and is not subject to first-pass metabolism.
US Natl Inst Health; DailyMed. Current Medication Information for AVIANE (levonorgestrel and ethinyl estradiol) kit (September 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14957
After a single dose of levonorgestrel and ethinyl estradiol 0.10 mg/0.02 mg tablets to 22 women under fasting conditions, maximum serum concentrations of levonorgestrel are 2.8 +/- 0.9 ng/mL (mean +/- SD) at 1.6 +/- 0.9 hours. At steady-state, attained from day 19 onwards, maximum levonorgestrel concentrations of 6 +/- 2.7 ng/mL are reached at 1.5 +/- 0.5 hours after the daily dose. The minimum serum levels of levonorgestrel at steady-state are 1.9 +/- 1 ng/mL. Observed levonorgestrel concentrations increased from day 1 (single dose) to days 6 and 21 (multiple doses) by 34% and 96%, respectively. Unbound levonorgestrel concentrations increased from day 1 to days 6 and 21 by 25% and 83%, respectively. The kinetics of total levonorgestrel are nonlinear due to an increase in binding of levonorgestrel to sex hormone binding globulin (SHBG), which is attributed to increased SHBG levels that are induced by the daily administration of ethinyl estradiol.
US Natl Inst Health; DailyMed. Current Medication Information for AVIANE (levonorgestrel and ethinyl estradiol) kit (September 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14957
The bioavailability of levonorgestrel is generally accepted to be 100%. This generalization is based on two studies that used only a small number of women. In one of the studies, absolute bioavailabilities were determined for doses of 0.25 and 0.15 mg levonorgestrel, each of which was administered to five women in combination with ethinylestradiol (0.05 mg). The results show that the bioavailability for the 0.15-mg dose of levonorgestrel ranged from 72 to 125% (mean, 99%); that for the 0.15-mg dose ranged from 63 to 108% (mean, 89%).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 147 (2007)
For more Absorption, Distribution and Excretion (Complete) data for LEVONORGESTREL (14 total), please visit the HSDB record page.
After absorption of the oral emergency contraceptive preparation, levonorgestrel is conjugated and forms a large number of sulfate conjugates. In addition, glucuronide conjugates have been identified in the plasma. High levels of conjugated and unconjugated 3, 5-tetrahydrolevonorgestrel are found in the plasma. The entire metabolic pathway for levonorgestrel has not been studied, however, 16-hydroxylation is one pathway that has been identified. Small quantities of 3, 5 tetrahydrolevonorgestrel and 16hydroxylevonorgestrel are also formed. No active metabolites have been identified. The rate of metabolism may be considerably different according to the patient and may explain a wide variation in levonorgestrel clearance. Liver CYP3A4 and CYP3A5 hepatic enzymes are reported to be involved in the metabolism of levonorgestrel.
(14)C-Norgestrel was administered to seven human subjects and 43% of dose was excreted in the urine within 5 days ... Enzymic hydrolysis released only 32% of the urinary radioactivity and a further 25% was excreted as sulphate conjugates. The metabolites excreted in the urine were much less polar than those following the administration of the related compounds, norethisterone or lynestrenol. The 3alphaOH,5beta and 3betaOH,5beta isomers of the tetrahydronorgestrel (13beta-ethyl-17alpha-ethynyl-5 beta-gonane-3alpha,17beta-diol) were isolated from urine and identified by mass spectrometry and thin-layer and gas-liquid chromatography. Plasma radioactivity decreased more rapidly than after the administration of norethisterone and lynestrenol. About 2% of the administered dose was converted to acidic compounds. There was no apparent difference in the rate of excretion of radioactivity or in the metabolites after either oral or intravenous administration of norgestrel.
Littleton P et al; Journal of Endocrinology 42: 591-598 (1968)
The comparative metabolism of dl-, d-, and l-norgestrel was investigated in African Green Monkeys (Cercopithecus aethiops). Total (14)C excretion in urine after a single oral dose of (14)C-dl-norgestrel (1 mg/kg) was significantly higher (51.4 +/- 5.0%) than that observed after administration of the d-enantiomer (37.5 +/- 5.4%) but not the l-enantiomer (44.2 +/- 8.9%). In all cases, the major part of the urinary radioactivity was present in a free fraction (48-62%), while an additional 13-27% was released by beta-glucuronidase preparations. No sulfate conjugates were detected. At least one major (16beta-hydroxylation) and one minor (16alpha-hydroxylation) metabolic pathway were stereoselective, i.e., they are operative with the I-but not the d-enantiomer. Three metabolites, 16beta-hydroxynorgestrel, 16alpha-hydroxynorgestrel, and 16-hydroxytetrahydronorgestrel (believed to be 16beta) were only detected in urine samples obtained from (14)C-dland -l-norgestrel-dosed animals. Following (14)C-d-norgestrel administration, 3alpha, 5beta-tetrahydronorgestrel was found to be the major urinary metabolite. These observations are compared with those reported earlier on the urinary metabolites of dl-norgestrel in women.
Sisenwine S et al; DMD 2 (1): 65-70 (1974)
The in vitro metabolism of stereo-isomers (d, l and the racemic mixture dl) of norgestrel by a microsomal fraction from rabbit liver was investigated. The metabolism of the biologically active l-norgestrel was more rapid than that of d-norgestrel (sic.) which is biologically inactive. This was mainly due to the more ready conversion of l-norgestrel to ring-A reduced metabolites. There was no difference between the two isomers in respect of the amount undergoing hydroxylation; about 40% of each isomer was converted to hydroxylated metabolites after 30 min incubation. However, there were differences between the isomers, l-norgestrel being converted mainly to the 16beta-hydroxysteroid and d-norgestrel to the 16alpha-hydroxysteroid. Similar amounts of both isomers were hydroxylated at C-6. The metabolism of the racemic mixture was intermediate between that of the d and l isomers.
Khana FS, Fotherby K; Journal of Steroid Biochemistry 19 (2): 1169-1172 (1983)
The rates of metabolism of synthetic gestagens derived from 19-nortestosterone by rabbit liver tissue in vitro were compared. Over a period of 1 hr norethisterone was metabolized as rapidly as 19-nortestosterone whereas d-norgestrel and lynestrenol were metabolized at a slightly lower rate. Less than 5% of l-norgestrel was metabolized. In all cases the reaction product was the tetrahydrosteroid. Lynestrenol was metabolised through norethisterone. Skeletal muscle, lung and small intestine also metabolized norethisterone and d-norgestrel but at a slower rate than liver tissue. Small amounts of norethisterone were metabolized by adipose tissue but heart and spleen were inactive. Lynestrenol and l-norgestrel were not metabolized by any of the extra-hepatic tissues studied.
Khana FS, Fotherby K; Journal of Steroid Biochemistry 10 (4): 437-442 (1978)
In vitro studies were conducted on the metabolism of 3 steroids used in OCs (oral contraceptives) by small pieces of human jejunal mucosa. This was done because the gastrointestinal mucosa of humans is known to metabolize a number of drugs. Almost 40% of the ethinyl estradiol, 9.8% of the levonorgestrel, and 7% of the mestranol were metabolized after incubation. All these metabolic responses were significantly different from those in the control groups. Results of the study show that the metabolism of the ethinyl estradiol was related to the weight of the tissue used. These results are consistent with the known marked 1st pass effect of ethinyl estradiol. Norgestrel, known to have little or no 1st pass effect, did not show a high rate of gut metabolism. Under the experimental conditions employed, no Phase 1 metabolism of either ethinyl estradiol or levonorgestrel was apparent.
PMID:6783058 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1401625 Back DJ et al; Br J Clin Pharmacol 11 (3): 275-8 (1981)
Following absorption, levonorgestrel is conjugated at the 17beta-OH position to form sulfate conjugates and, to a lesser extent, glucuronide conjugates in plasma. Significant amounts of conjugated and unconjugated 3alpha, 5beta-tetrahydrolevonorgestrel are also present in plasma, along with much smaller amounts of 3alpha, 5alpha-tetrahydrolevonorgestrel and 16beta-hydroxylevonorgestrel. Levonorgestrel and its phase I metabolites are excreted primarily as glucuronide conjugates. Metabolic clearance rates may differ among individuals by several-fold, and this may account in part for the wide variation observed in levonorgestrel concentrations among users.
US Natl Inst Health; DailyMed. Current Medication Information for NEXT CHOICE (levonorgestrel) tablet (December 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14292
The most important metabolic pathway for levonorgestrel occurs in the reduction of the delta4- and the 3-oxo-group as well as hydroxylations at positions 2alpha , 1beta, and 16beta, followed by conjugation. Most of the metabolites that circulate in the blood are sulfates of 3beta , 5beta -tetrahydro-levonorgestrel, while excretion occurs predominantly in the form of glucuronides. ... In-vitro studies on the biotransformation of levonorgestrel in human skin did not indicate any significant metabolism of levonorgestrel during skin penetration.
US Natl Inst Health; DailyMed. Current Medication Information for CLIMARA PRO (estradiol and levonorgestrel) patch (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=34043
/Investigators/ reviewed the metabolism of levonorgestrel in women treated orally with the radioactively labelled compound. Levonorgestrel was found mostly untransformed in serum within 1-2 hr after administration, but the concentrations of conjugated metabolites increased progressively between 4 and 24 hr after ingestion. Most of the conjugates were sulfates and glucuronides. In addition to the remaining unconjugated levonorgestrel, considerable amounts of unconjugated and sulfate-conjugated forms of 3alpha,5beta- tetrahydrolevonorgestrel were found; smaller quantities of conjugated and unconjugated 3alpha,5beta-tetrahydrolevonorgestrel and 16beta-hydroxylevonorgestrel were also identified. ... The major urinary metabolites were glucuronides (the most abundant was 3alpha,5beta-tetrahydrolevonorgestrel glucuronide) and smaller quantities of sulfates were found.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 146 (2007)
In vitro studies were conducted on the metabolism of 3 steroids used in OCs (oral contraceptives) by small pieces of human jejunal mucosa. This was done because the gastrointestinal mucosa of humans is known to metabolize a number of drugs. Almost 40% of the ethinyl estradiol, 9.8% of the levonorgestrel, and 7% of the mestranol were metabolized after incubation. All these metabolic responses were significantly different from those in the control groups. Results of the study show that the metabolism of the ethinyl estradiol was related to the weight of the tissue used. These results are consistent with the known marked 1st pass effect of ethinyl estradiol. Norgestrel, known to have little or no 1st pass effect, did not show a high rate of gut metabolism. Under the experimental conditions employed, no Phase 1 metabolism of either ethinyl estradiol or levonorgestrel was apparent.
PMID:6783058 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1401625 Back DJ et al; Br J Clin Pharmacol 11 (3): 275-8 (1981)
For more Metabolism/Metabolites (Complete) data for LEVONORGESTREL (6 total), please visit the HSDB record page.
The elimination half-life of a 0.75 mg dose of 1.5 mg of levonorgestrel ranges between 20-60 hours post-administration. A pharmacokinetic study of women with a normal BMI and BMI over revealed an elimination half-life of 29.7 h and 41.0-46.4 hours, respectively. Another pharmacokinetic study revealed a mean elimination half-life of 24.4 hours after a 0.75 mg dose of levonorgestrel was administered to 16 patients.
(14)C-Norgestrel was administered to seven human subjects and 43% of dose was excreted in the urine within 5 days; the biological half-life of the radioactivity was 24 hr. ...
Littleton P et al; Journal of Endocrinology 42: 591-598 (1968)
The elimination half-life for levonorgestrel is approximately 36 +/- 13 hours at steady-state.
US Natl Inst Health; DailyMed. Current Medication Information for AVIANE (levonorgestrel and ethinyl estradiol) kit (September 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14957
Mean (+/- SD) terminal half-life for levonorgestrel was determined to be 28 +/- 6.4 hours.
US Natl Inst Health; DailyMed. Current Medication Information for CLIMARA PRO (estradiol and levonorgestrel) patch (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=34043
... The mean half-life of elimination was found to be 13.2 hr and 9.9 hr for the 0.15-mg and 0.25-mg doses of levonorgestrel, respectively, when administered intravenously. These values were similar after oral dosing.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 147 (2007)
**Mechanism of action on ovulation** Oral contraceptives containing levonorgestrel suppress gonadotropins, inhibiting ovulation. Specifically, levonorgestrel binds to progesterone and androgen receptors and slows the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. This process results in the suppression of the normal physiological luteinizing hormone (LH) surge that precedes ovulation. It inhibits the rupture of follicles and viable egg release from the ovaries. Levonorgestrel has been proven to be more effective when administered before ovulation. **Mechanism of action in cervical mucus changes** Similar to other levonorgestrel-containing contraceptives, the intrauterine (IUD) forms of levonorgestrel likely prevent pregnancy by increasing the thickness of cervical mucus, interfering with the movement and survival of sperm, and inducing changes in the endometrium, where a fertilized ovum is usually implanted. Levonorgestrel is reported to alter the consistency of mucus in the cervix, which interferes with sperm migration into the uterus for fertilization. Levonorgestrel is not effective after implantation has occurred. Interestingly, recent evidence has refuted the commonly believed notion that levonorgestrel changes the consistency of cervical mucus when it is taken over a short-term period, as in emergency contraception. Over a long-term period, however, levonorgestrel has been proven to thicken cervical mucus. The exact mechanism of action of levonorgestrel is not completely understood and remains a topic of controversy and ongoing investigation. *Effects on implantation** The effects of levonorgestrel on endometrial receptivity are unclear, and the relevance of this mechanism to the therapeutic efficacy of levonorgestrel is contentious. Prescribing information for levonorgestrel IUDs state that they exert local morphological changes to the endometrium (e.g. stromal pseudodecidualization, glandular atrophy) that may play a role in their contraceptive activity. **Mechanism of action in hormone therapy** When combined with estrogens for the treatment of menopausal symptoms and prevention of osteoporosis, levonorgestrel serves to lower the carcinogenic risk of unopposed estrogen therapy via the inhibition of endometrial proliferation. Unregulated endometrial proliferation sometimes leads to endometrial cancer after estrogen use.
Combination oral contraceptives act by suppression of gonadotrophins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cer-vical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which may reduce the likelihood of implantation).
US Natl Inst Health; DailyMed. Current Medication Information Low-ogestrel (Norgestrel and Ethinyl Estradiol) (March 2007). Available from, as of March 29, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=4626
Progestins enter target cells by passive diffusion and bind to cytosolic (soluble) receptors that are loosely bound in the nucleus. The steroid receptor complex initiates transcription, resulting in an increase in protein synthesis. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
Progestins are capable of affecting serum concentrations of other hormones, particularly estrogen. Estrogenic effects are modified by the progestins, either by reducing the availability or stability of the hormone receptor complex or by turning off specific hormone-responsive genes by direct interaction with the progestin receptor in the nucleus. In addition, estrogen priming is necessary to increase progestin effects by upregulating the number of progestin receptors and/or increasing progesterone production, causing a negative feedback mechanism that inhibits estrogen receptors. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
There is great concern over the long-term influence of oral contraceptives on the development of breast cancer in women. Estrogens are known to stimulate the growth of human breast cancer cells, and /it/ has previously reported that the 19-norprogestin norethindrone could stimulate the proliferation of MCF-7 human breast cancer cells. /Investigators/ studied the influence of the 19-norprogestins norgestrel and gestodene compared to a 'non' 19-norprogestin medroxyprogesterone acetate (MPA) on MCF-7 cell proliferation. The 19-norprogestins stimulated proliferation at a concentration of 10(-8) M, while MPA could not stimulate proliferation at concentrations as great as 3 x 10(-6) M. The stimulatory activity of the 19-norprogestins could be blocked by the antioestrogen ICI 164,384, but not by the antiprogestin RU486. Transfection studies with the reporter plasmids containing an estrogen response element or progesterone response element (vitERE-CAT, pS2ERE-CAT, and PRE15-CAT) were performed to determine the intracellular action of norgestrel and gestodene. The 19-norprogestins stimulated the vitERE-CAT activity maximally at 10(-6) M, and this stimulation was inhibited by the addition of ICI 164,384. MPA did not stimulate vitERE-CAT activity. A single base pair alteration in the palindromic sequence of vitERE (resulting in the pS2ERE) led to a dramatic decrease in CAT expression by the 19-norprogestins, suggesting that the progestin activity required specific response element base sequencing. PRE15-CAT activity was stimulated by norgestrel, gestodene and MPA at concentrations well below growth stimulatory activity. This stimulation could be blocked by RU486. These studies suggest that the 19-norprogestins norgestrel and gestodene stimulate MCF-7 breast cancer cell growth by activating the estrogen receptor.
PMID:8494728 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1968434 Catherino WH et al; Br J Cancer 67 (5): 945-52 (1993)
Emergency contraceptive pills are not effective if a woman is already pregnant. Levonorgestrel is believed to act as an emergency contraceptive principally by preventing ovulation or fertilization (by altering tubal transport of sperm and/or ova). In addition, they may inhibit implantation (by altering the endometrium). It is not effective once the process of implantation has begun.
US Natl Inst Health; DailyMed. Current Medication Information for NEXT CHOICE (levonorgestrel) tablet (December 2009). Available from, as of February 5, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=14292
The precise mechanism of contraceptive activity of levonorgestrel administered after intercourse (postcoital) is not known. Levonorgestrel has been shown to inhibit or delay ovulation; other mechanisms of action for preventing pregnancy presumably are involved. Levonorgestrel is only effective before pregnancy is established. Once implantation occurs (ie, usually within 6-7 days after ovulation), levonorgestrel is ineffective in preventing pregnancy.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3122
Potential noncontraceptive benefits of oral contraceptives, as evidenced from epidemiologic studies, include effects on menses, effects related to inhibition of ovulation, and effects from long-term use. Use of the drugs has been associated with improved menstrual cycle regularity and decreased incidences of blood loss, iron deficiency anemia, and dysmenorrhea. A decreased incidence of functional ovarian cysts and of ectopic pregnancies also has been associated with use of the drugs. Long-term use of oral contraceptives has been associated with a decreased incidence of formation of fibroadenomas and fibrocystic disease of the breast, a decreased incidence of some (eg, gonococcal) pelvic inflammatory disease, and a decreased incidence of some cancers (eg, endometrial or ovarian cancer).
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3116
Progestins enter target cells by passive diffusion and bind to cytosolic (soluble) receptors that are loosely bound in the nucleus. The steroid receptor complex initiates transcription, resulting in an increase in protein synthesis. /Progestins/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 20th ed. Volume I. Micromedex, Inc. Englewood, CO., 2000. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2568
For more Mechanism of Action (Complete) data for LEVONORGESTREL (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
61
PharmaCompass offers a list of Levonorgestrel API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Levonorgestrel manufacturer or Levonorgestrel supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Levonorgestrel manufacturer or Levonorgestrel supplier.
PharmaCompass also assists you with knowing the Levonorgestrel API Price utilized in the formulation of products. Levonorgestrel API Price is not always fixed or binding as the Levonorgestrel Price is obtained through a variety of data sources. The Levonorgestrel Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Norgestrel manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Norgestrel, including repackagers and relabelers. The FDA regulates Norgestrel manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Norgestrel API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Norgestrel manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Norgestrel supplier is an individual or a company that provides Norgestrel active pharmaceutical ingredient (API) or Norgestrel finished formulations upon request. The Norgestrel suppliers may include Norgestrel API manufacturers, exporters, distributors and traders.
click here to find a list of Norgestrel suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Norgestrel DMF (Drug Master File) is a document detailing the whole manufacturing process of Norgestrel active pharmaceutical ingredient (API) in detail. Different forms of Norgestrel DMFs exist exist since differing nations have different regulations, such as Norgestrel USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Norgestrel DMF submitted to regulatory agencies in the US is known as a USDMF. Norgestrel USDMF includes data on Norgestrel's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Norgestrel USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Norgestrel suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Norgestrel Drug Master File in Japan (Norgestrel JDMF) empowers Norgestrel API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Norgestrel JDMF during the approval evaluation for pharmaceutical products. At the time of Norgestrel JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Norgestrel suppliers with JDMF on PharmaCompass.
A Norgestrel CEP of the European Pharmacopoeia monograph is often referred to as a Norgestrel Certificate of Suitability (COS). The purpose of a Norgestrel CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Norgestrel EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Norgestrel to their clients by showing that a Norgestrel CEP has been issued for it. The manufacturer submits a Norgestrel CEP (COS) as part of the market authorization procedure, and it takes on the role of a Norgestrel CEP holder for the record. Additionally, the data presented in the Norgestrel CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Norgestrel DMF.
A Norgestrel CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Norgestrel CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Norgestrel suppliers with CEP (COS) on PharmaCompass.
A Norgestrel written confirmation (Norgestrel WC) is an official document issued by a regulatory agency to a Norgestrel manufacturer, verifying that the manufacturing facility of a Norgestrel active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Norgestrel APIs or Norgestrel finished pharmaceutical products to another nation, regulatory agencies frequently require a Norgestrel WC (written confirmation) as part of the regulatory process.
click here to find a list of Norgestrel suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Norgestrel as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Norgestrel API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Norgestrel as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Norgestrel and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Norgestrel NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Norgestrel suppliers with NDC on PharmaCompass.
Norgestrel Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Norgestrel GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Norgestrel GMP manufacturer or Norgestrel GMP API supplier for your needs.
A Norgestrel CoA (Certificate of Analysis) is a formal document that attests to Norgestrel's compliance with Norgestrel specifications and serves as a tool for batch-level quality control.
Norgestrel CoA mostly includes findings from lab analyses of a specific batch. For each Norgestrel CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Norgestrel may be tested according to a variety of international standards, such as European Pharmacopoeia (Norgestrel EP), Norgestrel JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Norgestrel USP).
