
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Compound 201 995
2. Compound 201-995
3. Compound 201995
4. Octreotide
5. Octreotide Acetate
6. Octreotide Acetate Salt
7. San 201 995
8. San 201-995
9. San 201995
10. Sandostatin
11. Sandostatine
12. Sandoz 201 995
13. Sandoz 201-995
14. Sandoz 201995
15. Sm 201 995
16. Sm 201-995
17. Sm 201995
18. Sms 201 995
19. Sms 201-995
20. Sms 201995
1. Sandostatin
2. 79517-01-4
3. Nsc672461
4. Sandostatin (tn)
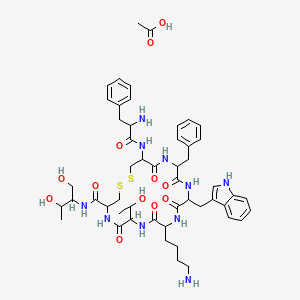
5. Acetic Acid;10-(4-aminobutyl)-19-[(2-amino-3-phenylpropanoyl)amino]-16-benzyl-n-(1,3-dihydroxybutan-2-yl)-7-(1-hydroxyethyl)-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide
6. Octreotide Acetate (usp)
7. Schembl58453
8. Chembl2000504
9. Hms3748c05
10. Bcp04661
11. Nsc671663
12. Nsc-671663
13. Nsc-672461
14. Acetic Acid;(4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-19-[[(2r)-2-amino-3-phenylpropanoyl]amino]-16-benzyl-n-[(2r,3r)-1,3-dihydroxybutan-2-yl]-7-[(1r)-1-hydroxyethyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide
15. D-phenylalanyl-l-cysteinyl-l-phenylalanyl-d-tryptophyl-l-lysyl-l-threonyl-n-[(1r,2r)-2-hydroxy-1-(hydroxymethyl)propyl]-l-cysteinamide Cyclic (2-7)-disulfide Acetate
16. 10-(4-aminobutyl)-19-((2-amino-3-phenylpropanoyl)amino)-16-benzyl-7-(1-hydroxyethyl)-n-(2-hydroxy-1-(hydroxymethyl)propyl)-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide Acetate
17. D06495
18. 10-(4-aminobutyl)-19-[(2-amino-3-phenyl-propanoyl)amino]-16-benzyl-7-(1-hydroxyethyl)-n-[2-hydroxy-1-(hydroxymethyl)propyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide
19. D-phenylalanyl-l-hemicystyl-l-phenylalanyl-d-trytophyl-l-lysyl-l-threonyl-l-hemicystyl-l-threoninol, Acetate
| Molecular Weight | 1079.3 g/mol |
|---|---|
| Molecular Formula | C51H70N10O12S2 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1078.46161005 g/mol |
| Monoisotopic Mass | 1078.46161005 g/mol |
| Topological Polar Surface Area | 420 Ų |
| Heavy Atom Count | 75 |
| Formal Charge | 0 |
| Complexity | 1780 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 10 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Octreotide acetate |
| Drug Label | Octreotide acetate injection, a cyclic octapeptide prepared as a clear sterile solution of octreotide, acetate salt, in a buffered acetate solution for administration by deep subcutaneous (intrafat) or intravenous injection. Octreotide acetate, known... |
| Active Ingredient | Octreotide acetate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.2mg base/ml; eq 1mg base/ml; eq 0.1mg base/ml; eq 0.05mg base/ml; eq 0.5mg base/ml |
| Market Status | Prescription |
| Company | Sun Pharm Inds; Teva Pharms Usa; Fresenius Kabi Usa; Sagent Pharms; Eurohlth Intl; Wockhardt Usa |
| 2 of 6 | |
|---|---|
| Drug Name | Sandostatin |
| PubMed Health | Octreotide (Injection) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Sandostatin (octreotide acetate) Injection, a cyclic octapeptide prepared as a clear sterile solution of octreotide, acetate salt, in a buffered lactic acid solution for administration by deep subcutaneous (intrafat) or intravenous injection. Octre... |
| Active Ingredient | Octreotide acetate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.2mg base/ml; eq 1mg base/ml; eq 0.1mg base/ml; eq 0.05mg base/ml; eq 0.5mg base/ml |
| Market Status | Prescription |
| Company | Novartis |
| 3 of 6 | |
|---|---|
| Drug Name | Sandostatin lar |
| Active Ingredient | Octreotide acetate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 30mg base/vial; eq 10mg base/vial; eq 20mg base/vial |
| Market Status | Prescription |
| Company | Novartis |
| 4 of 6 | |
|---|---|
| Drug Name | Octreotide acetate |
| Drug Label | Octreotide acetate injection, a cyclic octapeptide prepared as a clear sterile solution of octreotide, acetate salt, in a buffered acetate solution for administration by deep subcutaneous (intrafat) or intravenous injection. Octreotide acetate, known... |
| Active Ingredient | Octreotide acetate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.2mg base/ml; eq 1mg base/ml; eq 0.1mg base/ml; eq 0.05mg base/ml; eq 0.5mg base/ml |
| Market Status | Prescription |
| Company | Sun Pharm Inds; Teva Pharms Usa; Fresenius Kabi Usa; Sagent Pharms; Eurohlth Intl; Wockhardt Usa |
| 5 of 6 | |
|---|---|
| Drug Name | Sandostatin |
| PubMed Health | Octreotide (Injection) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Sandostatin (octreotide acetate) Injection, a cyclic octapeptide prepared as a clear sterile solution of octreotide, acetate salt, in a buffered lactic acid solution for administration by deep subcutaneous (intrafat) or intravenous injection. Octre... |
| Active Ingredient | Octreotide acetate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.2mg base/ml; eq 1mg base/ml; eq 0.1mg base/ml; eq 0.05mg base/ml; eq 0.5mg base/ml |
| Market Status | Prescription |
| Company | Novartis |
| 6 of 6 | |
|---|---|
| Drug Name | Sandostatin lar |
| Active Ingredient | Octreotide acetate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 30mg base/vial; eq 10mg base/vial; eq 20mg base/vial |
| Market Status | Prescription |
| Company | Novartis |
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
 Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
 Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Certificate Number : R0-CEP 2020-229 - Rev 01
Status : Valid
Issue Date : 2022-07-18
Type : Chemical
Substance Number : 2414
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2021-365 - Rev 00
Status : Valid
Issue Date : 2022-10-26
Type : Chemical
Substance Number : 2414

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2021-253 - Rev 00
Status : Valid
Issue Date : 2022-03-14
Type : Chemical
Substance Number : 2414

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2021-279 - Rev 00
Status : Valid
Issue Date : 2022-04-29
Type : Chemical
Substance Number : 2414

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2022-302 - Rev 00
Status : Valid
Issue Date : 2024-06-21
Type : Chemical
Substance Number : 2414

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF

Certificate Number : CEP 2022-031 - Rev 01
Status : Valid
Issue Date : 2024-07-22
Type : Chemical
Substance Number : 2414

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]  Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Registration Number : 306MF10083
Registrant's Address : Hauptstrasse 144,4416 Bubendorf Switzerland
Initial Date of Registration : 2024-07-03
Latest Date of Registration : 2024-07-03
Registration Number : 226MF10055
Registrant's Address : Viale dei Laboratori 54, 20092 Cinisello Balsamo (Milano) ITALY
Initial Date of Registration : 2014-03-06
Latest Date of Registration : 2014-03-06

Registration Number : 227MF10055
Registrant's Address : 1-4-29 Shibashima, Higashiyodogawa-ku, Osaka City, Osaka Prefecture
Initial Date of Registration : 2015-02-18
Latest Date of Registration : 2015-02-18

Registration Number : 226MF10062
Registrant's Address : SUN HOUSE, Plot No. 201 B/1, Western Express Highway, Goregaon (E), Mumbai 400063, Ma...
Initial Date of Registration : 2014-03-06
Latest Date of Registration : 2014-03-06

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2022-06-27
Valid Till : 2025-06-02
Written Confirmation Number : WC-0012
Address of the Firm : MIs USV Private Limited ,B-1/8,MIDC, Lote Parshuram, Tal., Dist. Ratnagiri, Khed...
Date of Issue : 2019-06-11
Valid Till : 2022-06-11
Written Confirmation Number : WC-0443
Address of the Firm : 4th Floor, Survey No. 71 & 72, Indrakaran (V), Kandi (M), Sangareddy (Dist.) Tel...

Date of Issue : 2019-09-03
Valid Till : 2022-09-02
Written Confirmation Number : WC-0237
Address of the Firm : C-43, MIDC, TTC Industrial Area, Turbhe, Off Thane Belapur Road, Dist: Thane Mah...

Date of Issue : 2019-06-27
Valid Till : 2022-06-26
Written Confirmation Number : WC-0047
Address of the Firm : K-28, MIDC, Anandnagar, Ambernath (E), Thane-421 506

Date of Issue : 2022-06-08
Valid Till : 2025-07-25
Written Confirmation Number : WC-0159
Address of the Firm : A-7/A-8, MIDC, Ahmednagar-414 111, Maharashtra State

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Meditip Co., Ltd.
Registration Date : 2022-07-11
Registration Number : 20220711-210-J-1331
Manufacturer Name : PolyPeptide Laboratories Pvt...
Manufacturer Address : Plot No. K28, Addtional MIDC, Anandnagar, Ambernath(E), Thane 421 506, Maharashtra St...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?