
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
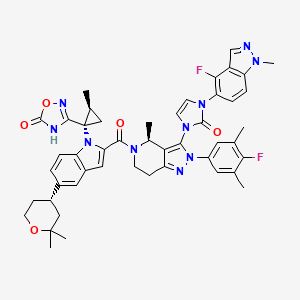

1. Ly3502970
1. 2212020-52-3
2. Ly3502970
3. Chembl4446782
4. Ly-3502970
5. 3-[(1s,2s)-1-[5-[(4s)-2,2-dimethyloxan-4-yl]-2-[(4s)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methylindazol-5-yl)-2-oxoimidazol-1-yl]-4-methyl-6,7-dihydro-4h-pyrazolo[4,3-c]pyridine-5-carbonyl]indol-1-yl]-2-methylcyclopropyl]-4h-1,2,4-oxadiazol-5-one
6. 3-[(1s,2s)-1-(5-[(4s)-2,2-dimethyloxan-4-yl]-2-{(4s)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methyl-1h-indazol-5-yl)-2-oxo-2,3-dihydro-1h-imidazol-1-yl]-4-methyl-2,4,6,7-tetrahydro-5h-pyrazolo[4,3-c]pyridine-5-carbonyl}-1h-indol-1-yl)-2-methylcyclopropyl]-1,2,4-oxadiazol-5(4h)-one
7. V6g
8. Orforglipron [inn]
9. Orforglipron [usan]
10. Ly3502970 (orforglipron)
11. Owl833
12. Schembl21175277
13. Gtpl12175
14. 7zw40d021m
15. Ex-a7751
16. Bdbm50514045
17. Akos040733262
18. Glp-1 Receptor Agonist 1;orforglipron
19. Ms-31635
20. Hy-112185
21. Cs-0043632
22. 1,2,4-oxadiazol-5(2h)-one, 3-[(1s,2s)-1-[2-[[(4s)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methyl-1h-indazol-5-yl)-2,3-dihydro-2-oxo-1h-imidazol-1-yl]-2,4,6,7-tetrahydro-4-methyl-5h-pyrazolo[4,3-c]pyridin-5-yl]carbonyl]-5-[(4s)-tetrahydro-2,2-dimethyl-2h-pyran-4-yl]-1h-indol-1-yl]-2-methylcyclopropyl]-
23. 3-[(1s,2s)-1-({2-(4-fluoro-3,5-dimethylphenyl)-3-({3-[3-(4-fluoro-1-methyl-1h-indazol-5-yl)-2-oxo-2,3-dihydro-1h-imidazol-1-yl]-4-methyl-2,4,6,7-tetrahydro-5h-pyrazolo[4,3-c]pyridin-5-yl}carbonyl)-5-[(4s)-2,2-dimethyloxan-4-yl]-1h-indol-1-yl}-2-methylcyclopropyl]-5-oxo-1,2,4-oxadiazol-4(5h)-ide
| Molecular Weight | 883.0 g/mol |
|---|---|
| Molecular Formula | C48H48F2N10O5 |
| XLogP3 | 6.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 144 |
| Heavy Atom Count | 65 |
| Formal Charge | 0 |
| Complexity | 1950 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
ABOUT THIS PAGE
