
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
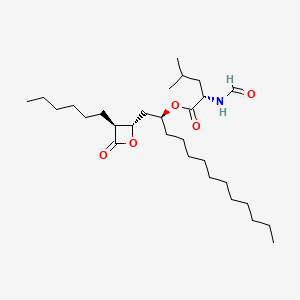

1. 1-((3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl-2-formamido-4-methylvalerate
2. Alli
3. Ro 18 0647
4. Ro-18-0647
5. Tetrahydrolipastatin
6. Tetrahydrolipstatin
7. Thlp
8. Xenical
1. 96829-58-2
2. Tetrahydrolipstatin
3. Xenical
4. Alli
5. Orlipastat
6. (-)-tetrahydrolipstatin
7. Orlipastatum [inn-latin]
8. Ro-18-0647
9. Ro 18-0647/002
10. (2s)-1-[(2s,3s)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl (2s)-2-formamido-4-methylpentanoate
11. [(2s)-1-[(2s,3s)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl] (2s)-2-formamido-4-methylpentanoate
12. N-formyl-l-leucine (1s)-1-[[(2s,3s)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl Ester
13. Orlistat (alli, Xenical)
14. Mls002207022
15. N-formyl-l-leucine, Ester With (3s,4s)-3-hexyl-4-((2s)-2-hydroxytridecyl)-2-oxetanone
16. Chembl175247
17. 95m8r751w8
18. Nsc-758881
19. Orlipastatum
20. Smr000466339
21. Thlp
22. Ro-180647002
23. Ro-180647-002
24. Ro-18-0647/002
25. (s)-1-((2s,3s)-3-hexyl-4-oxooxetan-2-yl)tridecan-2-yl Formyl-l-leucinate
26. L-leucine, N-formyl-, (1s)-1-(((2s,3s)-3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl Ester
27. Tetrahydrolipastatin
28. Mfcd05662360
29. (s)-((s)-1-((2s,3s)-3-hexyl-4-oxooxetan-2-yl)tridecan-2-yl) 2-formamido-4-methylpentanoate
30. L-leucine, N-formyl-, (1s)-1-[[(2s,3s)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl Ester
31. Xenical (tn)
32. Sr-01000759417
33. Orlistatum
34. Orlistat [usan:inn:ban]
35. Unii-95m8r751w8
36. Hsdb 7556
37. N-formyl-l-leucine (1s)-1-{[(2s,3s)-3-hexyl-4-oxo-2-oxetanyl]methyl}dodecyl Ester
38. Thl
39. Ks-1183
40. Lipase Inhibitor, Thl
41. Orlistat [hsdb]
42. Orlistat [usan]
43. Orlistat [inn]
44. Orlistat [jan]
45. Orlistat [mi]
46. (-)-tetrahydrolipostatin
47. Orlistat [vandf]
48. R-212
49. Orlistat [mart.]
50. Orlistat [usp-rs]
51. Orlistat [who-dd]
52. Orlistat (jan/usp/inn)
53. Orlistat [ema Epar]
54. Orlistat, >=98%, Solid
55. Schembl16408
56. L-leucine, N-formyl-, 1-((3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl Ester, (2s-(2alpha(r*),3beta))-
57. Mls000759448
58. Mls001423955
59. Bidd:gt0853
60. Orlistat [orange Book]
61. Gtpl5277
62. Dtxsid8023395
63. Orlistat [usp Monograph]
64. Bdbm24567
65. Chebi:94686
66. Tetrahydrolipstatin;ro-18-0647
67. Hms2051i08
68. Hms3413p06
69. Hms3677p06
70. Hy-b0218
71. Zinc8214635
72. S1629
73. Akos015894875
74. Bcp9001031
75. Ccg-100851
76. Db01083
77. Nc00101
78. Nsc 758881
79. Ro18-0647
80. (2s)-1-[(2s,3s)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl N-formyl-l-leucinate
81. Ncgc00165856-01
82. Ncgc00165856-02
83. Ncgc00165856-15
84. [(1s)-1-[[(2s,3s)-3-hexyl-4-oxo-oxetan-2-yl]methyl]dodecyl] (2s)-2-formamido-4-methyl-pentanoate
85. Bo164179
86. R212
87. Bcp0726000044
88. O0381
89. Sw197481-2
90. D04028
91. Ab00639987-09
92. Ab00639987_10
93. 829o582
94. Q424163
95. Q-201519
96. Sr-01000759417-5
97. Sr-01000759417-7
98. Z2379810072
99. Orlistat, United States Pharmacopeia (usp) Reference Standard
100. Orlistat, Pharmaceutical Secondary Standard; Certified Reference Material
101. 2-formamido-3-[(3-hexyl-4-oxo-oxetan-2-yl)methyl]-2-isobutyl-tetradecanoate
102. N-formyl-l-leucine (s)-1-[[(2s,3s)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl Ester
103. N-formyl-l-leucine-(s)-1-[[(2s,3s)-3-hexyl-4-oxo-2-oxetanyl]methyl]-dodecyl Ester
104. (2s)-2-formamido-4-methylpentanoic Acid [(2s)-1-[(2s,3s)-3-hexyl-4-oxo-2-oxetanyl]tridecan-2-yl] Ester
105. [(2s)-1-[(2r,3s)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl] (2r)-2-formamido-4-methylpentanoate
106. 104872-04-0
107. L-leucine, N-formyl-, 1-((3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl Ester, (2s-(2.alpha.(r*),3.beta.))-
| Molecular Weight | 495.7 g/mol |
|---|---|
| Molecular Formula | C29H53NO5 |
| XLogP3 | 10 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 23 |
| Exact Mass | 495.39237379 g/mol |
| Monoisotopic Mass | 495.39237379 g/mol |
| Topological Polar Surface Area | 81.7 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 579 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Alli |
| PubMed Health | Orlistat (By mouth) |
| Drug Classes | Dietary Fat Absorption Inhibitor |
| Active Ingredient | Orlistat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 60mg |
| Market Status | Over the Counter |
| Company | Glaxosmithkline Cons |
| 2 of 4 | |
|---|---|
| Drug Name | Xenical |
| PubMed Health | Orlistat (By mouth) |
| Drug Classes | Dietary Fat Absorption Inhibitor |
| Drug Label | XENICAL (orlistat) is a gastrointestinal lipase inhibitor for obesity management that acts by inhibiting the absorption of dietary fats.Orlistat is (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl e... |
| Active Ingredient | Orlistat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 120mg |
| Market Status | Prescription |
| Company | Hoffmann La Roche |
| 3 of 4 | |
|---|---|
| Drug Name | Alli |
| PubMed Health | Orlistat (By mouth) |
| Drug Classes | Dietary Fat Absorption Inhibitor |
| Active Ingredient | Orlistat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 60mg |
| Market Status | Over the Counter |
| Company | Glaxosmithkline Cons |
| 4 of 4 | |
|---|---|
| Drug Name | Xenical |
| PubMed Health | Orlistat (By mouth) |
| Drug Classes | Dietary Fat Absorption Inhibitor |
| Drug Label | XENICAL (orlistat) is a gastrointestinal lipase inhibitor for obesity management that acts by inhibiting the absorption of dietary fats.Orlistat is (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl e... |
| Active Ingredient | Orlistat |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 120mg |
| Market Status | Prescription |
| Company | Hoffmann La Roche |
Anti-Obesity Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
The Food and Drug Administration (FDA) today-(February 07, 2007) approved orlistat capsules as an over-the-counter (OTC) weight loss aid for overweight adults. Orlistat was initially approved in 1999 as a prescription drug to treat obesity, and remains a prescription drug for obesity at a higher dose than the OTC version. OTC orlistat will be manufactured by GlaxoSmithKline under the name Alli and is indicated for use in adults ages 18 years and older along with a reduced-calorie, low-fat diet, and exercise program.
FDA; FDA News. FDA Approves Orlistat for Over-the-Counter Use (P07-15). Available from, as of November 6, 2007: https://www.fda.gov/bbs/topics/NEWS/2007/NEW01557.html
Orlistat is indicated for the management of obesity in persons with an initial body mass index (BMI) greater than or equal to 30 kg per square meter of body surface area (kg/sq m), or a BMI greater than or equal to 27 kg/sq m when other risk factors (such as hypertension, diabetes, or dyslipidemia are present. Orlistat should be use in conjunction with a reduced calorie diet for management of obesity, including weight loss, weight maintenance, and reduction of the risk of weight gain following previous weight loss. Weight loss has been observed within 2 week of initiation or orlistat therapy. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2224
Chronic malabsorption syndrome or cholestasis /are contraindications for orlistat therapy/
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3020
/Orlistat is contraindicated in patients with/ known hypersensitivity to orlistat or any ingredient in the formulation.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3020
Caution /is advised/ in patients with a history of hyperoxaluria or calcium oxalate nephrolithiasis.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3020
/Clinician should/ rule out organic causes of obesity (e.g., hypothyroidism) /before initiating treatment/.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3020
For more Drug Warnings (Complete) data for ORLISTAT (12 total), please visit the HSDB record page.
Orlistat is indicated for obesity management including weight loss and weight maintenance when used in combination with calorie reduction in overweight and obese adults; this indication applies to both the prescription formulation of 120 mg and the over the counter formulation of 60 mg. Orlistat in the 120 mg prescription formulation is also indicated to reduce the risk for weight regain following weight loss.
FDA Label
Xenical is indicated in conjunction with a mildly hypocaloric diet for the treatment of obese patients with a body mass index (BMI) greater or equal to 30 kg/m2, or overweight patients (BMI > 28 kg/m2) with associated risk factors.
Treatment with orlistat should be discontinued after 12 weeks if patients have been unable to lose at least 5% of the body weight as measured at the start of therapy.
Orlistat helps with weight reduction and maintenance by inhibiting the absorption of dietary fats via the inhibition of lipase enzymes.
Anti-Obesity Agents
Agents that increase energy expenditure and weight loss by neural and metabolic regulation. (See all compounds classified as Anti-Obesity Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Lipid Regulating Agents
Substances that alter the metabolism of LIPIDS. (See all compounds classified as Lipid Regulating Agents.)
A08AB01
A08AB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A08 - Antiobesity preparations, excl. diet products
A08A - Antiobesity preparations, excl. diet products
A08AB - Peripherally acting antiobesity products
A08AB01 - Orlistat
Absorption
The systemic absorption and exposure of orlistat is low, however, systemic absorption of the drug is not required for orlistat activity. After an oral dose with 360 mg of radiolabeled orlistat, plasma radioactivity achieved a peak at about 8 hours. Plasma concentrations of unchanged parent drug were close to the lower end of detection limits (<5 ng/mL). In plasma samples of patients taking orlistat, the detection of unchanged drug was sporadic and very low concentrations were detected (<10 ng/mL or 0.02 M) with no evidence suggesting drug accumulation.
Route of Elimination
After single oral dose of radiolabled orlistat in both normal weight and obese volunteers fecal excretion of the unabsorbed drug was found to be the major route of elimination with <2% urinary excretion. Fecal elimination of orlistat is estimated between 95-97%. Complete excretion by both routes occurs within in 3 to 5 days.
Volume of Distribution
Volume of distribution cannot be obtained because the absorption of orlistat is minimal. Orlistat is minimally distributed to erythrocytes and is primarily bound to proteins.
Orlistat works locally within the GI tract, and therefore systemic absorption of the drug is not required for activity. In fact, systemic absorption of orlistat is minimal, and effects on systemic lipases are unlikely. Fecal excretion of unabsorbed drug is the major route of elimination.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3020
Systemic exposure to orlistat is minimal. Following oral dosing with 360 mg 14C-orlistat, plasma radioactivity peaked at approximately 8 hours; plasma concentrations of intact orlistat were near the limits of detection (<5 ng/mL). In therapeutic studies involving monitoring of plasma samples, detection of intact orlistat in plasma was sporadic and concentrations were low (<10 ng/mL or 0.02 uM), without evidence of accumulation, and consistent with minimal absorption.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2831
The average absolute bioavailability of intact orlistat was assessed in studies with male rats at oral doses of 150 and 1000 mg/kg/day and in male dogs at oral doses of 100 and 1000 mg/kg/day and found to be 0.12%, 0.59% in rats and 0.7%, 1.9% in dogs, respectively.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2831
In vitro orlistat was >99% bound to plasma proteins (lipoproteins and albumin were major binding proteins). Orlistat minimally partitioned into erythrocytes.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2831
For more Absorption, Distribution and Excretion (Complete) data for ORLISTAT (6 total), please visit the HSDB record page.
Orlistat is hydrolyzed in the intestinal wall. In a radiolabeled orlistat mass balance study in obese patients, two metabolites were identified. The first metabolite, M1, was the hydrolyzed -lactone ring product of orlistat. The second metabolite, M3, was produced from M1s cleavage of the N-formyl leucine side-chain. Both metabolites accounted for about 42% of total plasma radioactivity. Both M1 and M3 are considered pharmacologically inactive.
Based on animal data, it is likely that the metabolism of orlistat occurs mainly within the gastrointestinal wall. Based on an oral 14C-orlistat mass balance study in obese patients, two metabolites, M1 (4-member lactone ring hydrolyzed) and M3 (M1 with N-formyl leucine moiety cleaved), accounted for approximately 42% of total radioactivity in plasma. M1 and M3 have an open beta-lactone ring and extremely weak lipase inhibitory activity (1000- and 2500-fold less than orlistat, respectively). In view of this low inhibitory activity and the low plasma levels at the therapeutic dose (average of 26 ng/mL and 108 ng/mL for M1 and M3, respectively, 2 to 4 hours after a dose), these metabolites are considered pharmacologically inconsequential. The primary metabolite M1 had a short half-life (approximately 3 hours) whereas the secondary metabolite M3 disappeared at a slower rate (half-life approximately 13.5 hours). In obese patients, steady-state plasma levels of M1, but not M3, increased in proportion to orlistat doses.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2831
The half-life of orlistat of the small amount of absorbed orlistat ranges between 1-2 hours.
Based on limited data, the half-life of the absorbed orlistat is in the range of 1 to 2 hours.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2831
Based on an oral 14C-orlistat mass balance study in obese patients, ... the primary metabolite M1 had a short half-life (approximately 3 hours) whereas the secondary metabolite M3 disappeared at a slower rate (half-life approximately 13.5 hours). In obese patients, steady-state plasma levels of M1, but not M3, increased in proportion to orlistat doses.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2831
Orlistat is a potent and selective inhibitor of various lipase enzymes responsible for the metabolism of fat. It acts in the gastrointestinal (GI) tract via covalent binding to the serine residues located on the active site of both gastric and pancreatic lipase. When orlistat is taken with food containing fat, it partially inhibits the hydrolysis of triglycerides. This decreases absorption of monoaclglycerides and free fatty acids, contributing to weight maintenance and weight loss.
Orlistat is a reversible inhibitor of lipases. It exerts its therapeutic activity in the lumen of the stomach and small intestine by forming a covalent bond with the active serine residue site of gastric and pancreatic lipases. The inactivated enzymes are thus unavailable to hydrolyze dietary fat in the form of triglycerides into absorbable free fatty acids and monoglycerides. As undigested triglycerides are not absorbed, the resulting caloric deficit may have a positive effect on weight control. Systemic absorption of the drug is therefore not needed for activity. At the recommended therapeutic dose ... orlistat inhibits dietary fat absorption by approximately 30%.
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 2831
Orlistat, a reversible inhibitor of gastric and pancreatic lipases, exhibits antiobesity and antilipemic activity. The drug also inhibits certain other (e.g., microbial, carboxylester [for hydrolysis of vitamin esters]) lipases. Orlistat is a synthetic derivative of naturally occurring lipstatin.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3020
Unlike most other currently available antiobesity agents, orlistat does not exert anorexigenic (appetite suppressant) effects. Instead, orlistat exerts its antiobesity effect by decreasing the absorption of dietary fats (triacylglycerols) in the intestinal lumen via inhibition of triglyceride hydrolysis; at recommended dosages, approximately one-third of dietary fat will not be absorbed. By preventing triglyceride hydrolysis, the drug decreases intestinal concentrations of absorbable free fatty acids and monoglycerides.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3020
Orlistat, an antiobesity drug, is cytostatic and cytotoxic to tumor cells. The antitumor activity of orlistat can be attributed to its ability to inhibit the thioesterase domain of fatty acid synthase (FAS). The objective of the present study was to test the effect of orlistat on endothelial cell proliferation and angiogenesis. Orlistat inhibits endothelial cell FAS, blocks the synthesis of fatty acids, and prevents endothelial cell proliferation. More significantly, orlistat inhibits human neovascularization in an ex vivo assay, which suggests that it may be useful as an antiangiogenic drug. The mechanism of these effects can be traced to the fact that orlistat prevents the display of the vascular endothelial growth factor (VEGF) receptor (VEGFR2/KDR/Flk1) on the endothelial cell surface. Thus, orlistat is an antiangiogenic agent with a novel mechanism of action.
PMID:17012255 Browne C, Hindmarsh E et al; FASEB J 20 (12): 2027-35 (2006)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
91
PharmaCompass offers a list of Orlistat API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Orlistat manufacturer or Orlistat supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Orlistat manufacturer or Orlistat supplier.
PharmaCompass also assists you with knowing the Orlistat API Price utilized in the formulation of products. Orlistat API Price is not always fixed or binding as the Orlistat Price is obtained through a variety of data sources. The Orlistat Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Orlistat manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Orlistat, including repackagers and relabelers. The FDA regulates Orlistat manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Orlistat API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Orlistat manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Orlistat supplier is an individual or a company that provides Orlistat active pharmaceutical ingredient (API) or Orlistat finished formulations upon request. The Orlistat suppliers may include Orlistat API manufacturers, exporters, distributors and traders.
click here to find a list of Orlistat suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Orlistat DMF (Drug Master File) is a document detailing the whole manufacturing process of Orlistat active pharmaceutical ingredient (API) in detail. Different forms of Orlistat DMFs exist exist since differing nations have different regulations, such as Orlistat USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Orlistat DMF submitted to regulatory agencies in the US is known as a USDMF. Orlistat USDMF includes data on Orlistat's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Orlistat USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Orlistat suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Orlistat Drug Master File in Korea (Orlistat KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Orlistat. The MFDS reviews the Orlistat KDMF as part of the drug registration process and uses the information provided in the Orlistat KDMF to evaluate the safety and efficacy of the drug.
After submitting a Orlistat KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Orlistat API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Orlistat suppliers with KDMF on PharmaCompass.
A Orlistat written confirmation (Orlistat WC) is an official document issued by a regulatory agency to a Orlistat manufacturer, verifying that the manufacturing facility of a Orlistat active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Orlistat APIs or Orlistat finished pharmaceutical products to another nation, regulatory agencies frequently require a Orlistat WC (written confirmation) as part of the regulatory process.
click here to find a list of Orlistat suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Orlistat as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Orlistat API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Orlistat as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Orlistat and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Orlistat NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Orlistat suppliers with NDC on PharmaCompass.
Orlistat Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Orlistat GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Orlistat GMP manufacturer or Orlistat GMP API supplier for your needs.
A Orlistat CoA (Certificate of Analysis) is a formal document that attests to Orlistat's compliance with Orlistat specifications and serves as a tool for batch-level quality control.
Orlistat CoA mostly includes findings from lab analyses of a specific batch. For each Orlistat CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Orlistat may be tested according to a variety of international standards, such as European Pharmacopoeia (Orlistat EP), Orlistat JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Orlistat USP).
