
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
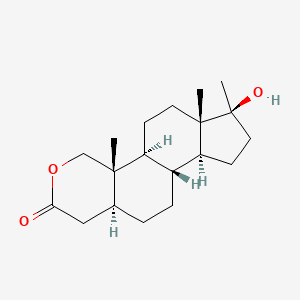

1. Anavar
2. Oxandrin
3. Sc 11585
4. Sc-11585
5. Sc11585
1. Anavar
2. Lonavar
3. Oxandrin
4. Vasorome
5. Provitar
6. 53-39-4
7. Ossandrolone [dcit]
8. Oxandrolonum
9. Oxandrolona
10. Oxandrolonum [inn-latin]
11. Oxandrolona [inn-spanish]
12. Protivar
13. Sc 11585
14. Sc-11585
15. Oxandrolone Ciii
16. (1s,3as,3br,5as,9as,9bs,11as)-1-hydroxy-1,9a,11a-trimethyl-2,3,3a,3b,4,5,5a,6,9,9b,10,11-dodecahydroindeno[4,5-h]isochromen-7-one
17. (4as,4bs,6as,7s,9as,9br,11as)-7-hydroxy-4a,6a,7-trimethyltetradecahydroindeno[4,5-h]isochromen-2(1h)-one
18. 7h6tm3ct4l
19. Chebi:7820
20. Nsc-67068
21. Ossandrolone
22. Oxandrin (tn)
23. 8075 C. B.
24. Hsdb 3373
25. Einecs 200-172-9
26. Nsc 67068
27. Unii-7h6tm3ct4l
28. 8075 Cb
29. 17beta-hydroxy-17alpha-methyl-2-oxa-5alpha-androstan-3-one
30. Oxandrolone (jan/usp/inn)
31. 17beta-hydroxy-17-methyl-2-oxa-5alpha-androstan-3-one
32. Oxandrin;anavar
33. 2-oxaandrostan-3-one, 17-hydroxy-17-methyl-, (5alpha,17beta)-
34. 17-beta-hydroxy-17-methyl-2-oxa-androstan-3-one
35. Dodecahydro-3-hydroxy-6-(hydroxy-methyl)-3,3a,6-trimethyl-1h-benz(e)indene-7-acetic Acid Delta-lactone
36. Oxandrolone [usan:usp:inn:ban:jan]
37. 2-oxa-5alpha-androstan-3-one, 17beta-hydroxy-17-methyl-
38. 8075 C.b.
39. Oxandrolone [mi]
40. Oxandrolone [inn]
41. Oxandrolone [jan]
42. Oxandrolone [hsdb]
43. Oxandrolone [usan]
44. Oxandrolone [vandf]
45. Oxandrolone [mart.]
46. Oxandrolone [who-dd]
47. 17.beta.-hydroxy-17-methyl-2-oxa-5.alpha.-androstan-3-one
48. Schembl148881
49. Gtpl7092
50. 2-oxaandrostan-3-one, 17-hydroxy-17-methyl-, (5.alpha.,17.beta.)-
51. Chembl1200436
52. Dtxsid8023399
53. Oxandrolone, >=98% (hplc)
54. Oxandrolone [orange Book]
55. Oxandrolone Ciii [usp-rs]
56. 2-oxa-5-alpha-androstan-3-one, 17-beta-hydroxy-17-methyl-
57. Hms3712e22
58. Oxandrolone [usp Monograph]
59. Hy-b0707
60. Zinc3813047
61. 2-oxaandrostan-3-one, 17-hydroxy-17-methyl-, (5-alpha,17-beta)-
62. Akos025401400
63. Ccg-220110
64. Db00621
65. Ac-14975
66. C07346
67. D00462
68. Ab01559948-01
69. 198o944
70. Q420859
71. Sr-01000872645
72. Sr-01000872645-1
73. W-105740
74. 17alpha-methyl-17beta-hydroxy-2-oxa-5alpha-androstan-3-one
75. (1s,2s,7s,10r,11s,14s,15s)-14-hydroxy-2,14,15-trimethyl-4-oxatetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-one
| Molecular Weight | 306.4 g/mol |
|---|---|
| Molecular Formula | C19H30O3 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 306.21949481 g/mol |
| Monoisotopic Mass | 306.21949481 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 503 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Oxandrin |
| PubMed Health | Oxandrolone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Oxandrin oral tablets contain 2.5 mg or 10 mg of the anabolic steroid oxandrolone. Oxandrolone is 17-hydroxy-17-methyl-2-oxa-5-androstan-3-one with the following structural formula:Inactive ingredients include cornstarch, lactose, magnesium s... |
| Active Ingredient | Oxandrolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 10mg |
| Market Status | Prescription |
| Company | Crealta Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Oxandrolone |
| PubMed Health | Oxandrolone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Oxandrolone Tablets, USP, oral tablets, contain 10 mg of the anabolic steroid oxandrolone. Oxandrolone is 17-hydroxy-17-methyl-2-oxa-5-androstan-3-one with the following structural formula:Inactive ingredients include anhydrous lactose, hyprome... |
| Active Ingredient | Oxandrolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 10mg |
| Market Status | Prescription |
| Company | Upsher Smith; Par Pharm |
| 3 of 4 | |
|---|---|
| Drug Name | Oxandrin |
| PubMed Health | Oxandrolone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Oxandrin oral tablets contain 2.5 mg or 10 mg of the anabolic steroid oxandrolone. Oxandrolone is 17-hydroxy-17-methyl-2-oxa-5-androstan-3-one with the following structural formula:Inactive ingredients include cornstarch, lactose, magnesium s... |
| Active Ingredient | Oxandrolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 10mg |
| Market Status | Prescription |
| Company | Crealta Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Oxandrolone |
| PubMed Health | Oxandrolone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent |
| Drug Label | Oxandrolone Tablets, USP, oral tablets, contain 10 mg of the anabolic steroid oxandrolone. Oxandrolone is 17-hydroxy-17-methyl-2-oxa-5-androstan-3-one with the following structural formula:Inactive ingredients include anhydrous lactose, hyprome... |
| Active Ingredient | Oxandrolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 10mg |
| Market Status | Prescription |
| Company | Upsher Smith; Par Pharm |
Anabolic Steroids
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Oxandrolone ... /is/ indicated in conditions such as chronic infections, extensive surgery, (corticosteroid-induced myopathy, decubitus ulcers, burns, /NOT included in US product labeling/) or severe trauma, which require reversal of catabolic processes or protein-sparing effects. /This agent is/ ... adjunct to, and not replacement for, conventional treatment of these disorders. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
Oxandrolone is used in the treatment of the short stature that accompanies Turner's syndrome (gonadal dysgenesis in females). Although the therapy is controversial, recent experimental reports seem to indicate that oxandrolone may be as effective as growth hormone and that oxandrolone may increase the efficacy of growth hormone therapy. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
Anabolic steroids may be used in children as an adjunct in the treatment of growth failure caused by pituitary growth hormone (GH) deficiency (pituitary dwarfism) or if the response to human growth hormone administration is inadequate. /Anabolic steroids; NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
For more Therapeutic Uses (Complete) data for OXANDROLONE (6 total), please visit the HSDB record page.
Peliosis hepatis, a condition in which liver and sometimes splenic tissue is replaced with blood-filed cysts, has occurred in patients receiving androgenic anabolic steroids. These cysts are sometimes present with minimal hepatic dysfunction and have been associated with liver failure. They are often not recognized until life-threatening liver failure or intro-abdominal hemorrhage develops. Withdrawal of drug usually results in complete disappearance of lesions. /Anabolic steroids/
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 334
Liver cell tumors: Most often these tumors are benign and androgen-dependent, but fatal malignant tumors have occurred. Withdrawal of drug often results in regression or cessation of tumor progression. However, hepatic tumors associated with androgens or anabolic steroids are much more vascular than other hepatic tumors and may be silent until life-threatening intra-abdominal hemorhage develops. /Anabolic steroids/
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 324
Use of anabolic steroids by athletes is not recommended. Objective evidence is conflicting and inconclusive as to whether these medications significantly increase athletic performance by increasing muscle strength. Weight gains reported by athletes are due in part to fluid retention, which is a potentially hazardous side effect of anabolic steroid therapy. The risk of other unwanted effects, such as testicular atrophy and suppression of spermatogenesis in males; menstrual disturbances and virilization, such as deepening of voice, development of acne, and unnatural growth of body hair in females; peliosis hepatis or other hepatotoxicity; and hepatic cancer outweigh and possible benefit received from anabolic steroids and make their use in athletes inappropriate. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 140
Anabolic steroids are not recommended for use during pregnancy, since studies in animals have shown that anabolic steroids cause masculinization of the fetus. Risk-benefit must be carefully considered. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
For more Drug Warnings (Complete) data for OXANDROLONE (22 total), please visit the HSDB record page.
Use to promote weight gain after weight loss following extensive surgery.
FDA Label
Oxandrolone is an anabolic steroids indicated as adjunctive therapy to promote weight gain after weight loss following extensive surgery, chronic infections, or severe trauma, and in some patients who without definite pathophysiologic reasons fail to gain or to maintain normal weight, to offset the protein catabolism associated with prolonged administration of corticosteroids, and for the relief of the bone pain frequently accompanying osteoporosis. Anabolic steroids are synthetic derivatives of testosterone.
Androgens
Compounds that interact with ANDROGEN RECEPTORS in target tissues to bring about the effects similar to those of TESTOSTERONE. Depending on the target tissues, androgenic effects can be on SEX DIFFERENTIATION; male reproductive organs, SPERMATOGENESIS; secondary male SEX CHARACTERISTICS; LIBIDO; development of muscle mass, strength, and power. (See all compounds classified as Androgens.)
Anabolic Agents
These compounds stimulate anabolism and inhibit catabolism. They stimulate the development of muscle mass, strength, and power. (See all compounds classified as Anabolic Agents.)
A - Alimentary tract and metabolism
A14 - Anabolic agents for systemic use
A14A - Anabolic steroids
A14AA - Androstan derivatives
A14AA08 - Oxandrolone
It is not known whether anabolic steroids are distributed into breast milk. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
Renal
The metabolism of 17 alpha-methyl-17 beta-hydroxy-2-oxa-5 alpha-androstan-3-one (oxandrolone) in man has been investigated by gas chromatography/mass spectrometry. After oral administration of a 10 mg dose to man, five metabolites were detected in the free fraction of the urinary samples. Oxandrolone, the major compound excreted in urine, was detected within 72 hr after administration. During this period 35.8 and 8.4% of the administered dose was excreted as unchanged oxandrolone and 17-epioxandrolone, respectively. In addition, minute amounts of 16 alpha- and 16 beta-hydroxyoxandrolone and a delta-hydroxy acid resulting from the hydrolysis of the lactone group of oxandrolone were detected in the urine samples 8-60 hr after administration.
PMID:2765703 Masse R et al; Biomed Environ Mass Spectrom 18 (6): 429-38 (1989)
0.55 hours (1st phage), 9 hours (2nd phase)
Biphasic: 1st phase - 0.55 hours. 2nd phase - 9 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
Oxandrolones interact with androgen receptors in target tissues.
Reverses catabolic processes and negative nitrogen balance by promoting protein anabolism and stimulating appetite if there is concurrently a proper intake of calories and proteins. /Anabolic steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
There was abs decrease in total serum cholesterol and redistribution of cholesterol, such that post-treatment low-density lipoprotein carried less cholesterol and high-density lipoprotein more cholesterol on percentage basis than found in pre-treatment values with oxandrolone.
FREEMAN MW ET AL; J GERONTOL 35(1) 31 (1980)
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2017-12-05
Pay. Date : 2017-07-28
DMF Number : 31920
Submission : 2017-10-28
Status : Active
Type : II

Date of Issue : 2024-02-20
Valid Till : 2026-12-06
Written Confirmation Number : WC-0416
Address of the Firm :

NDC Package Code : 58159-046
Start Marketing Date : 2024-08-14
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (35kg/35kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20344
Submission : 2007-03-06
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17962
Submission : 2005-01-05
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16598
Submission : 2003-05-22
Status : Active
Type : II

NDC Package Code : 55486-1570
Start Marketing Date : 2012-06-03
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34723
Submission : 2020-03-17
Status : Active
Type : II

NDC Package Code : 64181-0003
Start Marketing Date : 2007-06-22
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (8kg/8kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16938
Submission : 2003-10-31
Status : Inactive
Type : II

NDC Package Code : 65129-1066
Start Marketing Date : 2001-05-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17459
Submission : 2004-06-09
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16217
Submission : 2002-10-21
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15427
Submission : 2001-05-17
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
68
PharmaCompass offers a list of Oxandrolone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Oxandrolone manufacturer or Oxandrolone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Oxandrolone manufacturer or Oxandrolone supplier.
PharmaCompass also assists you with knowing the Oxandrolone API Price utilized in the formulation of products. Oxandrolone API Price is not always fixed or binding as the Oxandrolone Price is obtained through a variety of data sources. The Oxandrolone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Oxandrolone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Oxandrolone, including repackagers and relabelers. The FDA regulates Oxandrolone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Oxandrolone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Oxandrolone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Oxandrolone supplier is an individual or a company that provides Oxandrolone active pharmaceutical ingredient (API) or Oxandrolone finished formulations upon request. The Oxandrolone suppliers may include Oxandrolone API manufacturers, exporters, distributors and traders.
click here to find a list of Oxandrolone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Oxandrolone DMF (Drug Master File) is a document detailing the whole manufacturing process of Oxandrolone active pharmaceutical ingredient (API) in detail. Different forms of Oxandrolone DMFs exist exist since differing nations have different regulations, such as Oxandrolone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Oxandrolone DMF submitted to regulatory agencies in the US is known as a USDMF. Oxandrolone USDMF includes data on Oxandrolone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Oxandrolone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Oxandrolone suppliers with USDMF on PharmaCompass.
A Oxandrolone written confirmation (Oxandrolone WC) is an official document issued by a regulatory agency to a Oxandrolone manufacturer, verifying that the manufacturing facility of a Oxandrolone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Oxandrolone APIs or Oxandrolone finished pharmaceutical products to another nation, regulatory agencies frequently require a Oxandrolone WC (written confirmation) as part of the regulatory process.
click here to find a list of Oxandrolone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Oxandrolone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Oxandrolone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Oxandrolone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Oxandrolone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Oxandrolone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Oxandrolone suppliers with NDC on PharmaCompass.
Oxandrolone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Oxandrolone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Oxandrolone GMP manufacturer or Oxandrolone GMP API supplier for your needs.
A Oxandrolone CoA (Certificate of Analysis) is a formal document that attests to Oxandrolone's compliance with Oxandrolone specifications and serves as a tool for batch-level quality control.
Oxandrolone CoA mostly includes findings from lab analyses of a specific batch. For each Oxandrolone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Oxandrolone may be tested according to a variety of international standards, such as European Pharmacopoeia (Oxandrolone EP), Oxandrolone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Oxandrolone USP).
