
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
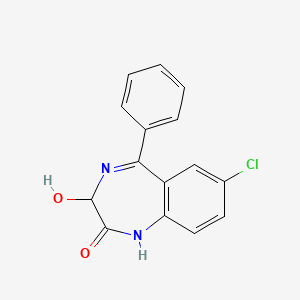

1. Adumbran
2. Serax
3. Tazepam
1. Serax
2. Tazepam
3. 604-75-1
4. Adumbran
5. Droxacepam
6. Zaxopam
7. Ansioxacepam
8. Anxiolit
9. Durazepam
10. Nesontil
11. Noctazepam
12. Praxiten
13. Psiquiwas
14. Quilibrex
15. Aplakil
16. Astress
17. Bonare
18. Drimuel
19. Isodin
20. Limbial
21. Murelax
22. Pacienx
23. Propax
24. Rondar
25. Sedigoa
26. Serenid
27. Serepax
28. Seresta
29. Serpax
30. Sobril
31. Vaben
32. Hi-long
33. Azutranquil
34. Psicopax
35. Sigacalm
36. Uskan
37. (rs)-oxazepam
38. Nortemazepam
39. Nozepam
40. Tarchomin
41. (+-)-oxazepam
42. Serenid-d
43. Oxazepamum
44. Oxozepam
45. Tacepam
46. Quen
47. Tranquo-buscopan-wirkstoff
48. Wy-3498
49. Z10-tr
50. Ro 5-6789
51. Cb 8092
52. 2h-1,4-benzodiazepin-2-one, 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-
53. 7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-2h-1,4-benzodiazepin-2-one
54. Oxazepam Civ
55. 7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
56. 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2h-1,4-benzodiazepin-2-one
57. 6gow6dwn2a
58. Nsc-169448
59. Chebi:7823
60. Abboxapam
61. Lederpam
62. Alepam
63. Oxanid
64. Anxiolit Retard
65. Oxa-puren
66. J3.308a
67. N-desmethyltemazepam
68. Ossazepam [dcit]
69. Ncgc00159323-02
70. Ncgc00159323-03
71. Ossazepam
72. Oxazipam
73. Dsstox_cid_1087
74. Oxazepamum [inn-latin]
75. Dsstox_rid_75934
76. Dsstox_gsid_21087
77. Z 10 Tr
78. Wy 3498
79. Cas-604-75-1
80. Ccris 488
81. Serax (tn)
82. Hsdb 3140
83. Einecs 210-076-9
84. Unii-6gow6dwn2a
85. Nsc 169448
86. Oxazepam (jan/usp/inn)
87. Dea No. 2835
88. 1,3-dihydro-7-chloro-3-hydroxy-5-phenyl-2h-1,4-benzodiazepin-2-one
89. Oxazepam [usan:usp:inn:ban:jan]
90. Oxazepam [hsdb]
91. Oxazepam [iarc]
92. Oxazepam [usan]
93. Oxazepam [inn]
94. Oxazepam [jan]
95. Oxazepam [mi]
96. Oxazepam [vandf]
97. (.+/-.)-oxazepam
98. Oxazepam [mart.]
99. Chembl568
100. Oxazepam [who-dd]
101. (+-)-7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2h-1,4-benzodiazepin-2-one
102. Oprea1_501459
103. Schembl27435
104. Temazepam Impurity, Oxazepam-
105. Divk1c_000986
106. Oxazepam [orange Book]
107. Oxazepam Civ [usp-rs]
108. Gtpl7253
109. Oxazepam [ep Monograph]
110. Dtxsid1021087
111. Niosh/df2371850
112. Oxazepam [usp Monograph]
113. Adimayptobdmtl-uhfffaoysa-
114. Bdbm85031
115. Hms503e13
116. Kbio1_000986
117. Oxazepam 0.1 Mg/ml In Methanol
118. Oxazepam 1.0 Mg/ml In Methanol
119. Ninds_000986
120. Nsc_4616
121. Tox21_111572
122. Tox21_112819
123. Tox21_200700
124. Tox21_303501
125. 1,3-dihydro-7-chloro-3-hydroxy-5-phenyl-3h-1,4-benzodiazepin-2-one
126. 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2h-1,4-benzodiazepine-2-one
127. 7-chloro-1,4-benzodiazepine-2-one
128. Mfcd00057903
129. Nsc169448
130. Akos016347293
131. Db00842
132. 2h-1,4-benzodiazepin-2-one, 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-, (+-)-
133. Idi1_000986
134. Wln: T67 Gmv Jn Ihj Cg Iq Kr
135. Ncgc00159323-04
136. Ncgc00257352-01
137. Ncgc00258254-01
138. Cas_604-75-1
139. Temazepam Impurity B [ep Impurity]
140. Df23718500
141. C07359
142. D00464
143. 604o751
144. Q412299
145. Sr-01000942251
146. 2h-1, 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-
147. 7-chloro-5-phenyl-3h-1,4-benzodiazepine-2,3-diol
148. Sr-01000942251-1
149. Temazepam Impurity, Oxazepam- [usp Impurity]
150. 7-chloro-3-hydroxy-5-phenyl-1,4-benzodiazepin-2-one
151. Oxazepam Solution, Drug Standard, 1.0 Mg/ml In Methanol
152. Oxazepam, European Pharmacopoeia (ep) Reference Standard
153. 3-hydroxy-1,3-dihydro-7-chloro-5-phenyl-2h-1,4-benzodiazepin-2-one
154. 3-hydroxy-5-phenyl-7-chloro-2,3-dihydro-1h-1,4-benzodiazepin-2-one
155. 3h-1,4-benzodiazepin-2-one, 1,3-dihydro-7-chloro-3-hydroxy-5-phenyl-
156. 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-1,4 [2hl-benzodiazepin-2-one
157. 7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-benzo[e][1,4]diazepin-2-one
158. 7-chloro-3-hydroxy-5-phenyl-2,3-dihydro-1h-1,4-benzodiazepin-2-one
159. (+/-)-7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2h-1,4-benzodiazepin-2-one
160. 2h-1,4-benzodiazepin-2-one, 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-, (+/-)-
161. 7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-2h-benzo[e][1,4]diazepin-2-one
162. Oxazepam For Peak Identification, European Pharmacopoeia (ep) Reference Standard
163. Oxazepam Solution, 1 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 286.71 g/mol |
|---|---|
| Molecular Formula | C15H11ClN2O2 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 286.0509053 g/mol |
| Monoisotopic Mass | 286.0509053 g/mol |
| Topological Polar Surface Area | 61.7 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 407 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Oxazepam |
| PubMed Health | Oxazepam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Oxazepam is the first of a chemical series of compounds, the 3-hydroxybenzodiazepinones. A therapeutic agent providing versatility and flexibility in control of common emotional disturbances, this product exerts prompt action in a wide variety of dis... |
| Active Ingredient | Oxazepam |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 15mg; 10mg |
| Market Status | Prescription |
| Company | Sandoz; Actavis Elizabeth |
| 2 of 2 | |
|---|---|
| Drug Name | Oxazepam |
| PubMed Health | Oxazepam (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Oxazepam is the first of a chemical series of compounds, the 3-hydroxybenzodiazepinones. A therapeutic agent providing versatility and flexibility in control of common emotional disturbances, this product exerts prompt action in a wide variety of dis... |
| Active Ingredient | Oxazepam |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 30mg; 15mg; 10mg |
| Market Status | Prescription |
| Company | Sandoz; Actavis Elizabeth |
Anti-Anxiety Agents, Benzodiazepine; GABA Modulators; Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
... Oxazepam /is/ indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. ... /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 518
... Oxazepam /is/ indicated for the adjunctive management of anxiety associated with mental depression. Effectiveness of ... /this/ medication for long-term use has not been assessed in systematic clinical studies. The medication's efficacy in an individual patient should be reassessed at periodic intervals. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 518
... Oxazepam ... /is/ indicated for the relief of acute alcohol withdrawal symptoms such as acute agitation, tremor, impending or acute delirium tremens, and hallucinosis. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 519
Late third trimester use and exposure during labor seems to be associated with much greater risks to the fetus and neonate. Some infants exposed at this time exhibit either the floppy infant syndrome or marked neonatal withdrawal symptoms. Symptoms vary from mild sedation, hypotonia, and reluctance to suck, to apneic spells, cyanosis, and impaired metabolic responses to cold stress. These symptoms have been reported to persist for periods from hours to months after birth. This correlates well with the pharmacokinetic and placental transfer of the benzodiazepines and their disposition in the neonate. /Benzodiazepines/
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 817
Potentially serious, adverse behavioral effects occasionally have been associated with benzodiazepine use. Such effects include confusion, bizarre or abnormal behavior, agitation, hyperexcitability, auditory and visual hallucinations, paranoid ideation, panic, delirium, agitation, sleepwalking, aggression and antisocial acts; in some cases, amnesia about the behavior may occur. ... /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2376
Benzodiazepine abuse is common and occurs with all benzodiazepines. ... /Benzodiazepines/
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 819
Patients should be warned that benzodiazepines may impair ability to perform hazardous activities requiring mental alertness or physical coordination (e.g., operating machinery, driving a motor vehicle). Patients also should be warned about possible effects on memory (anterograde amnesia) and to report promptly to their physician any behavioral or mental change, including disturbing thought and unusual manners of conduct, that develops during benzodiazepine therapy. Benzodiazepines should be used with caution and large quantities of the drugs should not be prescribed for patient with suicidal tendencies or whose history indicates that they may increase dosage on their own initiative. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2376
For more Drug Warnings (Complete) data for OXAZEPAM (16 total), please visit the HSDB record page.
Oxazepam is indicated for the management of anxiety disorders and for the short-term relief of symptoms of anxiety. It may also be used in the management of alcohol withdrawal symptoms.
Benzodiazepines, including oxazepam, exert their sedative and anxiolytic effects by potentiating the effects of endogenous GABA, the primary inhibitory neurotransmitter in the CNS. Compared to other benzodiazepines, it has relatively low potency and a moderate duration of action. Oxazepam should be administered with caution to patients for whom a drop in blood pressure may lead to cardiac complications as, in rare cases, it may cause hypotension.
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
N05BA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA04 - Oxazepam
Absorption
Following oral administration, peak plasma levels (Cmax) averaged 450 mg/mL and occurred approximately 3 hours (Tmax) after dosing.
Route of Elimination
Oxazepam is primarily eliminated in the urine as its glucuronide metabolite, with the feces containing approximately 21% of the unchanged drug. The majority of an orally ingested dose of oxazepam is excreted within 48 hours.
The miniature swine (like humans) eliminated oxazepam primarily as the glucuronides, while aromatic hydroxylation predominated in the rat. In rats, 70.7 +/- 6.0% of a single oral dose of 20 mg/kg bw was eliminated in feces following biliary secretion, while 18.9 +/- 2.4% of the dose was found in the urine. In CD-1 mice given an oral dose of 22 mg/kg bw oxazepam, 57.8% was recovered from the feces and 27.3% was recovered from urine over five days. ... Treatment with 2500 mg/kg diet (ppm) oxazepam in the diet for 14 days before administration of oxazepam by gastric instillation led to a shift from fecal to urinary excretion in mice, but not rats, so that the urinary excretion almost doubled.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 126 (1996)
Oxazepam accumulates in adipose tissue. /It was/ found that adipose tissue/blood ratios of the drug in mice given 5 mg/kg bw intravenously varied from 1.7 (at 5 min) to 4.9 (at 30 min). Accumulation also occurred in the brain. Maximal concentrations of oxazepam in the brain were 14.3 +/- 0.17 ug/g in mice, 4.5 +/- 0.03 ug/g in rats and 3.5 +/- 0.47 ug/g in guinea-pigs, all at 5 min. Brain/blood drug level ratios in these species varied from 1.1 (at 1 min) to 11.3 (at 10 hr) in mice, from 1.9 (at 1 min) to 6.2 (at 1 hr) in rats and from 1.9 (at 5 min) to 8.9 (at 5 hr) in guinea-pigs.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 126 (1996)
/It was/ found that the time of maximum absorption of 30 mg oxazepam was 2.2 hr (range, 0.75-4.25 hr) in 18 men and 3.1 hr (range, 0.5-8.0 hr) in 20 women. The maximal plasma concentrations in this study were 622 +/- 37 ng/mL in men and 837 +/- 51 ng/mL in women.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 123 (1996)
Oxazepam is absorbed fairly rapidly, reaching peak plasma concentrations within 1-4 hr, with a mean of about 2 hr in most studies.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 123 (1996)
The plasma concentrations of oxazepam in male B6C3F1 mice fed diet containing 125 and 2500 mg/kg (ppm) oxazepam appeared to reach steady-state levels by one week of feeding. These levels were 1 ug/mL for the low-dose group and 5-10 ug/mL for the high-dose group
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 124 (1996)
Oxazepam has a single major inactive metabolite, a glucuronide conjugate. The glucuronidation of the S-isomer is catalyzed by UGT2B15. The glucuronidation of the R-isomer is catalyzed by UGT2B7 and UGT1A9.
The metabolism and the anticonvulsant effect of clorazepate were followed for 2 h after its i.v. administration to mice. The ED50 of the drug was 12 mg/kg at 1 min against pentetrazole-induced convulsions (45 mg/kg i.v.), it reached a minimum at 1 hr (2.0 mg/kg) and rose to 2.7 mg/kg at 2 h. The concentrations of unchanged clorazepate and its metabolites, desmethyldiazepam and oxazepam, were determined in plasma and brain after administration of the respective ED50s. Unchanged clorazepate could be detected in plasma for the first hour but never in brain, so it can be considered as inactive pro-drug. The brain concentrations of desmethyldiazepam and oxazepam after the respective ED50s of clorazepate were considerably higher at 1 and 15 min than after longer time intervals. This may be explained by a time lag needed to reach and bind to the benzodiazepine receptor.
PMID:2908106 Frey HH, Scherki R; Eur J Pharmacol 158 (3): 213-6 (1988)
... Oxazepam ... /is/ metabolized by direct conjugation with glucuronic acid.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 520
Oxazepam is a commonly used 1,4-benzodiazepine anxiolytic drug that is polymorphically metabolized in humans. However, the molecular basis for this phenomenon is currently unknown. We have previously shown that S-oxazepam glucuronide, the major oxazepam metabolite, is selectively formed by UDP-glucuronosyltransferase (UGT) 2B15, whereas the minor Roxazepam glucuronide is produced by multiple UGTs other than UGT2B15. Phenotype-genotype studies were conducted using microsomes and DNA prepared from the same set of 54 human livers. Sequencing of the UGT2B15 gene revealed three nonsynonymous polymorphisms, D85Y, T352I, and K523T, with variant allele frequencies of 0.56, 0.02, and 0.40, respectively. D85Y genotype showed a significant effect (p = 0.012) on S-oxazepam glucuronidation with lower median activities in 85Y/Y livers (49 pmol/min/mg protein) compared with 85D/D livers (131 pmol/min/mg), whereas 85D/Y livers were intermediate in activity (65 pmol/min/mg). There was also a significant trend (p = 0.049) for higher S-oxazepam activities in the two 352T/I livers (135 and 210 pmol/min/mg) compared with the remaining 352T/T livers (median, 64 pmol/min/mg). Conversely, K523T genotype had no apparent effect on oxazepam glucuronidation (p > 0.05). Donor gender also significantly influenced S-oxazepam glucuronidation with higher median activities in male (65 pmol/min/mg) compared with female (39 pmol/min/ mg) livers (p = 0.042). R-Oxazepam glucuronidation was not affected by either genotype or gender (p > 0.05). In conclusion, gender and D85Y genotype are identified as major determinants of S-oxazepam glucuronidation by human liver and may explain in part polymorphic oxazepam glucuronidation by human subjects.
PMID:15044558 Court MH et al; J Pharmacol Exp Ther 310 (2): 656-65 (2004)
There are three major pathways of oxazepam metabolism in mice and rats (as in humans): direct conjugation, phenyl ring oxidation and diazepine ring contraction. In mice, conjugation is mainly with glucuronide, predominantly excreted in the urine; in rats, conjugation is mainly with sulfate, which is almost entirely eliminated in the feces. The sulfate conjugate of oxazepam, which is unstable in acidic media, may be the source of the fecal oxazepam It has not been detected in mice. Studies with recirculating, perfused male Swiss (CD-1) mouse liver preparations showed that oxazepam glucuronides are the dominant liver metabolites in this species. Oxazepam can also be conjugated with glucuronide by the placenta of rabbits, apparently in contrast to the human organ. Phenyl ring oxidation is more important in rats than in mice (or humans) and a dihydrodiol (probably the 3',4'- dihydrodiol, since 2'-hydroxy derivatives are not known) accounts for about 30% of the 72-hr urinary metabolites in Fischer 344 rats. This metabolite, which probably forms via an arene oxide intermediate and has not been found in mice, holds implications for the toxicological properties of oxazepam. In rats, ring contraction to 6-chloro-4-phenyl- 2(1H)-quinazoline carboxylic acid occurs to roughly one half of the extent seen in mice.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 126 (1996)
A method for the extraction of diazepam and its metabolites (nordiazepam, temazepam, and oxazepam) from equine urine and serum and their quantitation and confirmation by liquid chromatography-tandem mass spectrometry is presented. Valium, a formulation of diazepam, was administered at a dose of 10 mg intramuscularly to four standard-bred mares. Diazepam is extensively metabolized in the horse to nordiazepam, temazepam, and oxazepam. Diazepam urinary concentrations were found to be less than 6 ng/mL. Nordiazepam was found to be mainly in its glucuronide-conjugated form and was measured out to a collection time of 53-55 hr. Oxazepam and temazepam were entirely conjugated, and their urinary concentrations were measured out to collection times of 121 hr and 77-79 hr, respectively. Diazepam and nordiazepam were measured in equine postadministration serum out to collection times of 6 and 54 hr, respectively. Oxazepam and temazepam were not detected in postadministration serum.
PMID:10022206 Marland A et al; J Anal Toxicol 23 (1): 29-34 (1999)
Oxazepam has known human metabolites that include Oxazepam glucuronide.
Oxazepam is a known human metabolite of nordiazepam and schembl29464.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean elimination half-life of oxazepam is 8.2 hours.
Considerable variation in the elimination half life has been reported, with mean values ranging from about 5 to about 15 hr. Values of 6.7 hr (range, 5.5-9.2 hr) and 5.8 hr (range, 5.4- 8.4 hr) /were found/ following intravenous and oral administration, respectively. A sex difference has been reported, with a value of 7.8 +/- 0.4 hr (range, 4.9-10.8 hr) in men and 9.7 +/- 0.8 hr (range, 6.3-19.4 hr) in women.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V66 123 (1996)
Elimination half-life of oxazepam is 3-21 hr. /From table/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2380
Like other benzodiazepines, oxazepam exerts its anxiolytic effects by potentiating the effect of gamma-aminobutyric acid (GABA) on GABA(A) receptors, the main inhibitory neurotransmitter receptors in the mammalian brain. GABA(A) receptors are a component of GABA-gated ionotropic chloride channels that produce inhibitory postsynaptic potentials - following activation by GABA, the channel undergoes a conformational change that allows the passage of chloride ions through the channel. The inhibitory potentials produced by GABA neurotransmission play an integral role in the suppression and control of epileptiform nerve firing such as that seen in epilepsy, which makes the GABA system a desirable target in the treatment of epilepsy. Benzodiazepines are positive allosteric modulators of GABA(A) function. They bind to the interface between alpha () and gamma () subunits on the receptor, commonly referred to as the benzodiazepine binding site, and modulate the receptor such that its inhibitory response to GABA binding is dramatically increased.
The inhibitory neurotransmitter, gamma-aminobutyric acid (GABA), acts on GABAA receptors to regulate vigilance, anxiety, muscle tension, epileptogenic activity and memory functions. Benzodiazepines modulate GABA-evoked chloride currents through a binding site on the GABAA receptor-operated chloride channel. GABA agonists and benzodiazepine agonists simultaneously enhance the binding of the other to its receptor. Benzodiazepine binding appears to shift the GABA receptor from a low affinity state to a high affinity state and also stabilizes the receptor in a conformation that permits the ion channel to remain open. Similarly, GABA binding also enhances benzodiazepine agonist binding to its receptor via the same mechanism. Thus, GABA receptor agonist and benzodiazepine receptor agonists are positive allosteric effectors for each other.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 816-7
The exact sites and mode of action of the benzodiazepines are unknown. The drugs appear to act at the limbic, thalamic, and hypothalamic levels of the CNS, producing anxiolytic, sedative, hypnotic, skeletal muscle relaxant, and anticonvulsant effects. The effects of benzodiazepines may be mediated through the inhibitory neurotransmitter gamma-aminobutyric acid. Benzodiazepines are capable of producing all levels of CNS depression from mild sedation to hypnosis to coma. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379
Anxiolytic and possibly paradoxical CNS stimulatory effects of benzodiazepines are postulated to result from release of previously suppressed responses (disinhibition). After usual doses of benzodiazepines for several days, the drugs cause a moderate decrease in rapid eye movement sleep. Rapid eye movement rebound does not occur when the drugs are withdrawn. Stage 3 and 4 sleep are markedly reduced by usual doses of the drugs; the clinical importance of these sleep stage alterations has not been established. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379
Benzodiazepines appear to produce skeletal muscle relaxation predominantly by inhibiting spinal polysynaptic afferent pathways, but the drugs may also inhibit monosynaptic afferent pathways. The drugs may inhibit monosynaptic and polysynaptic reflexes by acting as inhibitory neuronal transmitters or by blocking exitatory synaptic transmission. The drugs may also directly depress motor nerve and muscle function. /Benzodiazepines/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2379

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
56
PharmaCompass offers a list of Oxazepam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Oxazepam manufacturer or Oxazepam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Oxazepam manufacturer or Oxazepam supplier.
PharmaCompass also assists you with knowing the Oxazepam API Price utilized in the formulation of products. Oxazepam API Price is not always fixed or binding as the Oxazepam Price is obtained through a variety of data sources. The Oxazepam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Oxazepam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Oxazepam, including repackagers and relabelers. The FDA regulates Oxazepam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Oxazepam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Oxazepam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Oxazepam supplier is an individual or a company that provides Oxazepam active pharmaceutical ingredient (API) or Oxazepam finished formulations upon request. The Oxazepam suppliers may include Oxazepam API manufacturers, exporters, distributors and traders.
click here to find a list of Oxazepam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Oxazepam DMF (Drug Master File) is a document detailing the whole manufacturing process of Oxazepam active pharmaceutical ingredient (API) in detail. Different forms of Oxazepam DMFs exist exist since differing nations have different regulations, such as Oxazepam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Oxazepam DMF submitted to regulatory agencies in the US is known as a USDMF. Oxazepam USDMF includes data on Oxazepam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Oxazepam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Oxazepam suppliers with USDMF on PharmaCompass.
A Oxazepam CEP of the European Pharmacopoeia monograph is often referred to as a Oxazepam Certificate of Suitability (COS). The purpose of a Oxazepam CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Oxazepam EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Oxazepam to their clients by showing that a Oxazepam CEP has been issued for it. The manufacturer submits a Oxazepam CEP (COS) as part of the market authorization procedure, and it takes on the role of a Oxazepam CEP holder for the record. Additionally, the data presented in the Oxazepam CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Oxazepam DMF.
A Oxazepam CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Oxazepam CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Oxazepam suppliers with CEP (COS) on PharmaCompass.
A Oxazepam written confirmation (Oxazepam WC) is an official document issued by a regulatory agency to a Oxazepam manufacturer, verifying that the manufacturing facility of a Oxazepam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Oxazepam APIs or Oxazepam finished pharmaceutical products to another nation, regulatory agencies frequently require a Oxazepam WC (written confirmation) as part of the regulatory process.
click here to find a list of Oxazepam suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Oxazepam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Oxazepam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Oxazepam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Oxazepam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Oxazepam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Oxazepam suppliers with NDC on PharmaCompass.
Oxazepam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Oxazepam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Oxazepam GMP manufacturer or Oxazepam GMP API supplier for your needs.
A Oxazepam CoA (Certificate of Analysis) is a formal document that attests to Oxazepam's compliance with Oxazepam specifications and serves as a tool for batch-level quality control.
Oxazepam CoA mostly includes findings from lab analyses of a specific batch. For each Oxazepam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Oxazepam may be tested according to a variety of international standards, such as European Pharmacopoeia (Oxazepam EP), Oxazepam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Oxazepam USP).
