
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada

Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
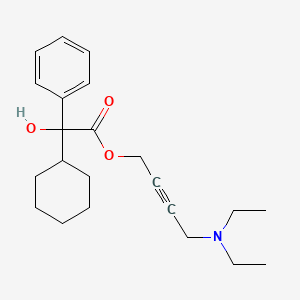

1. 4-(diethylamino)-2-butynyl-alpha-cyclohexyl-alpha-hydroxybenzeneacetate
2. 4-(diethylamino)-2-butynyl-alpha-phenylcyclohexaneglycolate
3. Apo-oxybutynin
4. Contimin
5. Cystonorm
6. Cystrin
7. Ditropan
8. Dresplan
9. Dridase
10. Driptane
11. Gelnique
12. Gen-oxybutynin
13. Novo-oxybutynin
14. Nu-oxybutyn
15. Oxyb Abz
16. Oxybugamma
17. Oxybutin Holsten
18. Oxybuton
19. Oxybutynin Al
20. Oxybutynin Azu
21. Oxybutynin Chloride
22. Oxybutynin Heumann
23. Oxybutynin Hexal
24. Oxybutynin Hydrochloride
25. Oxybutynin Stada
26. Oxybutynin Von Ct
27. Oxybutynin-puren
28. Oxybutynin-ratiopharm
29. Oxymedin
30. Oxytrol
31. Pms-oxybutynin
32. Pollakisu
33. Renamel
34. Ryol
35. Spasmex Oxybutynin
36. Spasyt
37. Tavor
38. Zatur
1. 5633-20-5
2. Ditropan
3. Oxytrol
4. Oxibutyninum
5. Kentera
6. Oxybutyninum
7. Oxybutinin
8. Oxybutynine
9. Oxibutinina
10. Oxybutynine [inn-french]
11. Oxybutyninum [inn-latin]
12. Oxibutinina [inn-spanish]
13. 4-diethylamino-2-butinyl Alpha-cyclohexylmandelat
14. Ditropan Xl
15. Oxybutynin (ditropan)
16. 4-(diethylamino)but-2-ynyl 2-cyclohexyl-2-hydroxy-2-phenylacetate
17. 4-(diethylamino)but-2-yn-1-yl 2-cyclohexyl-2-hydroxy-2-phenylacetate
18. 4-diethylamino-2-butynyl Alpha-phenylcyclohexaneglycolate
19. Chembl1231
20. Chebi:7856
21. 4-(diethylamino)-2-butynyl Alpha-phenylcyclohexaneglycolic Acid Ester
22. K9p6mc7092
23. 5633-20-5 (free)
24. Cyclohexaneglycolic Acid, Alpha-phenyl-, 4-(diethylamino)-2-butynyl Ester
25. Benzeneacetic Acid, Alpha-cyclohexyl-alpha-hydroxy-, 4-(diethylamino)-2-butynyl Ester
26. Cystrin
27. 4-(diethylamino)but-2-yn-1-yl Cyclohexyl(hydroxy)phenylacetate
28. Oxybutynin Base
29. Anturol
30. Transdermal Patch
31. Lyrinel Xl
32. Oxytrol For Women
33. Oxybutynin [usan:inn:ban]
34. Ccris 1923
35. Oxybutynin Transdermal
36. Oxybutynin Topical Gel
37. Hsdb 3270
38. Oxybutynin Transdermal Patch
39. Oxybutinyn
40. Unii-k9p6mc7092
41. (rs)-oxybutynin
42. Anturol (tn)
43. Oxytrol (tn)
44. Mfcd00865252
45. Oxybutynin [mi]
46. Oxybutynin [inn]
47. Oxybutynin (usan/inn)
48. Prestwick0_000287
49. Prestwick1_000287
50. Prestwick2_000287
51. Prestwick3_000287
52. Oxybutynin [hsdb]
53. Oxybutynin [usan]
54. Oxybutynin [vandf]
55. Oxybutynin [mart.]
56. Schembl2992
57. Oxybutynin [who-dd]
58. Lopac0_000923
59. Bspbio_000194
60. Gtpl359
61. Mls006010052
62. Oxybutynin [ema Epar]
63. Spbio_002413
64. Bpbio1_000214
65. Dtxsid0023406
66. Oxybutynin [orange Book]
67. Oxybutynin, Oxybutynin Chloride
68. Chebi:144551
69. Hms3884k08
70. Bcp12179
71. Hy-b0267
72. Ab7701
73. Bdbm50165019
74. S1754
75. Akos015896242
76. Cyclohexaneglycolic Acid, .alpha.-phenyl-, 4-(diethylamino)-2-butynyl Ester
77. Ac-2153
78. Ccg-205005
79. Db01062
80. Ks-5221
81. Sdccgsbi-0050898.p003
82. Benzeneacetic Acid, .alpha.-cyclohexyl-.alpha.-hydroxy-, 4-(diethylamino)-2-butynyl Ester
83. Mrf-0000601
84. (+/-)-oxybutynin(hydrochloride Form)
85. Ncgc00015767-03
86. Ncgc00015767-04
87. Ncgc00015767-06
88. Ncgc00015767-07
89. Ncgc00015767-09
90. Ncgc00015767-10
91. Ncgc00015767-21
92. Ncgc00089795-02
93. Smr001550466
94. Sbi-0050898.p002
95. Ft-0603679
96. Sw196787-3
97. C07360
98. D00465
99. 633o205
100. A831004
101. L000923
102. Q1060922
103. Brd-a65013509-003-03-8
104. Brd-a65013509-003-13-7
105. 4-(diethylamino)-2-butynyl .alpha.-phenylcyclohexaneglycolate
106. 4-(diethylamino)-2-butynyl Cyclohexyl(hydroxy)phenylacetate #
107. 4-diethylaminobut-2-ynyl 2-cyclohexyl-2-hydroxy-2-phenylacetate
108. Rac-4-(diethylamino)but-2-yn-1-yl Cyclohexyl(hydroxy)phenylacetate
109. (r)-4-(diethylamino)but-2-ynyl 2-cyclohexyl-2-hydroxy-2-phenylacetate
110. (2r)-2-cyclohexyl-2-hydroxy-2-phenylacetic Acid 4-(diethylamino)but-2-ynyl Ester
111. 4-(diethylamino)-2-butynyl .alpha.-phenylcyclohexaneglycolic Acid Ester
112. Alpha-cyclohexyl-alpha-hydroxybenzeneacetic Acid 4-(diethylamino)-2-butynyl Ester
113. Benzeneacetic Acid, A-cyclohexyl-a-hydroxy-,4-(diethylamino)-2-butynyl Ester
114. 119579-36-1
| Molecular Weight | 357.5 g/mol |
|---|---|
| Molecular Formula | C22H31NO3 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 357.23039385 g/mol |
| Monoisotopic Mass | 357.23039385 g/mol |
| Topological Polar Surface Area | 49.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 490 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Oxybutynin |
| PubMed Health | Oxybutynin |
| Drug Classes | Urinary Antispasmodic |
| Drug Label | Oxybutynin chloride tablets USP are a debossed, very pale blue, round, biconvex, scored tablet containing 5 mg of oxybutynin chloride, USP. Chemically, oxybutynin chloride is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate hydrochlor... |
| Active Ingredient | Oxybutynin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 3.9mg/24hr |
| Market Status | Prescription |
| Company | Barr Labs Div Teva |
| 2 of 4 | |
|---|---|
| Drug Name | Oxytrol |
| PubMed Health | Oxybutynin (Absorbed through the skin) |
| Drug Classes | Urinary Antispasmodic |
| Drug Label | OXYTROL (oxybutynin transdermal system) is designed to deliver oxybutynin over a 3- to 4-day interval after application to intact skin. OXYTROL is available as a 39 cm2 system containing 36 mg of oxybutynin. OXYTROL has a nominal in vivo delivery rat... |
| Active Ingredient | Oxybutynin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 3.9mg/24hr |
| Market Status | Prescription |
| Company | Watson Labs (utah) |
| 3 of 4 | |
|---|---|
| Drug Name | Oxybutynin |
| PubMed Health | Oxybutynin |
| Drug Classes | Urinary Antispasmodic |
| Drug Label | Oxybutynin chloride tablets USP are a debossed, very pale blue, round, biconvex, scored tablet containing 5 mg of oxybutynin chloride, USP. Chemically, oxybutynin chloride is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate hydrochlor... |
| Active Ingredient | Oxybutynin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 3.9mg/24hr |
| Market Status | Prescription |
| Company | Barr Labs Div Teva |
| 4 of 4 | |
|---|---|
| Drug Name | Oxytrol |
| PubMed Health | Oxybutynin (Absorbed through the skin) |
| Drug Classes | Urinary Antispasmodic |
| Drug Label | OXYTROL (oxybutynin transdermal system) is designed to deliver oxybutynin over a 3- to 4-day interval after application to intact skin. OXYTROL is available as a 39 cm2 system containing 36 mg of oxybutynin. OXYTROL has a nominal in vivo delivery rat... |
| Active Ingredient | Oxybutynin |
| Dosage Form | Film, extended release |
| Route | Transdermal |
| Strength | 3.9mg/24hr |
| Market Status | Prescription |
| Company | Watson Labs (utah) |
Parasympatholytics; Cholinegeric Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
OXYBUTYNIN IS AN ANTISPASMODIC USED IN TREATMENT OF UNINHIBITED OR REFLEX-TYPE NEUROGENIC BLADDER. ON BASIS OF ANIMAL PHARMACOLOGIC STUDIES, IT IS THOUGHT TO HAVE BOTH ANTICHOLINERGIC AND MUSCULOTROPIC PROPERTIES. /CHLORIDE/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1200
...OXYBUTYNIN IMPROVED SYMPTOMS OF NEUROGENIC BLADDER SUCH AS URINARY URGENCY, URGE INCONTINENCE, NOCTURIA, & PAIN. ...IN CHILDREN WITH ENURESIS, THERE WAS REDN IN INCIDENCE OF BEDWETTING, URINARY FREQUENCY, & DAYTIME ACCIDENTS. ... ITS EFFECTIVENESS IS COMPARABLE TO THAT OF OTHER ANTISPASMODIC AGENTS... /CHLORIDE/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1200
Oxybutynin is indicated for the symptomatic treatment of overactive bladder, which causes urge urinary incontinence and frequency, and urgency. Oxybutynin may also be used for children aged 6 and above for the symptomatic management of detrusor muscle overactivity which has been found to be related to a neurological condition. Spina bifida is an example of a neurological condition in which oxybutynin may be used to control urinary symptoms. On occasion, oxybutynin may be used off-label to relieve bladder spasms associated with ureteral stents or urinary catheters.
Symptomatic treatment of urge incontinence and/or increased urinary frequency and urgency as may occur in adult patients with unstable bladder.
Oxybutynin exerts antispasmodic actions on the bladder, relieving the uncomfortable symptoms of overactive bladder, including urinary urgency and frequency. These actions occur through the inhibition of muscarinic receptors. **A note on angioedema and anticholinergic effects** Symptoms of angioedema may occur with oxybutynin therapy. If angioedema is suspected, discontinue oxybutynin immediately and provide appropriate medical treatment. In addition, anticholinergic effects may occur with the administration of this drug. Some symptoms may include hallucinations, confusion, agitation, and drowsiness. It is advisable to avoid operating heavy machinery before the response to oxybutynin has been monitored. Dose adjustments may be required.
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
G04BD04
G04BD04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BD - Drugs for urinary frequency and incontinence
G04BD04 - Oxybutynin
Absorption
Oxybutynin should be swallowed whole with the help of liquids. A pharmacokinetic study revealed that oxybutynin was rapidly absorbed, and peak concentrations were reached within about 1 hour of administration, measured at 8.2 ngml-1 and AUC was 16 ngml-1. The biovailability of oxybutynin is about 6%, and the plasma concentration of the active metabolite, desethyloxybutynin is 5 to 12 times greater than that of oxybutynin. Bioavailability is increased in the elderly. Food has been shown to increase the exposure to controlled-release oxybutynin.
Route of Elimination
Oxybutynin is heavily cleared by the liver. Under 0.1% of an administered dose is found as unchanged drug in the urine. Less than 0.1% of a single dose of oxybutynin is excreted as desethyloxybutynin.
Volume of Distribution
Oxybutynin has a wide volume of distribution of 193 L. In rats, oxybutynin penetrates the central nervous system.
Oxybutynin is heavily metabolized by the CYP3A4 enzyme system in both the liver and the wall of the intestine. It undergoes first-pass metabolism, and its resulting primary active metabolite, N-desethyloxybutynin circulates. It is active at the muscarinic receptors in both the bladder and the salivary gland. Hepatic biotransformation also produces its major inactive metabolite, phenylcyclohexylglycolic acid.
The plasma elimination half-life is about 2 hours. In the elderly, the elimination half-life is prolonged up to 5 hours.
Oxybutynin acts to relax the bladder by inhibiting the muscarinic action of acetylcholine on smooth muscle, and not skeletal muscle. The active of oxybutynin is metabolite is N-desethyloxybutynin. It competitively inhibits the postganglionic type 1, 2 and 3 muscarinic receptors. The above actions lead to increased urine capacity in the bladder, decreasing urinary urgency and frequency. In addition, oxybutynin delays the initial desire to void.
RESULTS OF CYSTOMETRIC STUDIES SHOWED THAT THE DRUG INCR BLADDER CAPACITY @ ONSET OF FIRST CONTRACTION & FIRST DESIRE TO VOID, AS WELL AS @ END OF CYSTOMETRY. /CHLORIDE/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1200

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
31
PharmaCompass offers a list of Oxybutynin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Oxybutynin manufacturer or Oxybutynin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Oxybutynin manufacturer or Oxybutynin supplier.
PharmaCompass also assists you with knowing the Oxybutynin API Price utilized in the formulation of products. Oxybutynin API Price is not always fixed or binding as the Oxybutynin Price is obtained through a variety of data sources. The Oxybutynin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Oxybutynin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Oxybutynin, including repackagers and relabelers. The FDA regulates Oxybutynin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Oxybutynin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Oxybutynin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Oxybutynin supplier is an individual or a company that provides Oxybutynin active pharmaceutical ingredient (API) or Oxybutynin finished formulations upon request. The Oxybutynin suppliers may include Oxybutynin API manufacturers, exporters, distributors and traders.
click here to find a list of Oxybutynin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Oxybutynin DMF (Drug Master File) is a document detailing the whole manufacturing process of Oxybutynin active pharmaceutical ingredient (API) in detail. Different forms of Oxybutynin DMFs exist exist since differing nations have different regulations, such as Oxybutynin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Oxybutynin DMF submitted to regulatory agencies in the US is known as a USDMF. Oxybutynin USDMF includes data on Oxybutynin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Oxybutynin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Oxybutynin suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Oxybutynin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Oxybutynin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Oxybutynin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Oxybutynin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Oxybutynin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Oxybutynin suppliers with NDC on PharmaCompass.
Oxybutynin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Oxybutynin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Oxybutynin GMP manufacturer or Oxybutynin GMP API supplier for your needs.
A Oxybutynin CoA (Certificate of Analysis) is a formal document that attests to Oxybutynin's compliance with Oxybutynin specifications and serves as a tool for batch-level quality control.
Oxybutynin CoA mostly includes findings from lab analyses of a specific batch. For each Oxybutynin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Oxybutynin may be tested according to a variety of international standards, such as European Pharmacopoeia (Oxybutynin EP), Oxybutynin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Oxybutynin USP).
