

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
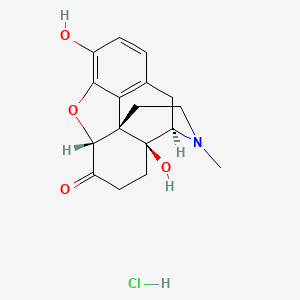

1. Numorphan
2. Opana
3. Oxymorphone
4. Oxymorphone Hcl
1. Oxymorphone Hcl
2. 357-07-3
3. Opana Er
4. Oxymorphone Hydrochloride [usp]
5. 5y2ei94nbc
6. Oxymorphone Hcl Narcotic Analgesic
7. Oxymorphone Hydrochloride (usp)
8. Oxymorphinone Hydrochloride
9. (4r,4as,7ar,12bs)-4a,9-dihydroxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;hydrochloride
10. Einecs 206-610-5
11. Unii-5y2ei94nbc
12. Numorphan (tn)
13. Opana (tn)
14. 4,5-alpha-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
15. 4,5alpha-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
16. Schembl41770
17. Chebi:7866
18. Chembl1200794
19. Dtxsid10189214
20. Oxymorphone Hydrochloride [mi]
21. (5alpha)-4,5-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
22. Morphinan-6-one, 3,14-dihydroxy-4,5-alpha-epoxy-17-methyl-, Hydrochloride
23. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, Hydrochloride, (5alpha)-
24. Oxymorphone Hydrochloride [mart.]
25. Oxymorphone Hydrochloride [vandf]
26. Oxymorphone Hydrochloride [who-dd]
27. Oxymorphone Hydrochloride [green Book]
28. D00844
29. Oxymorphone Hydrochloride [orange Book]
30. Oxymorphone Hydrochloride [usp Monograph]
31. Q27263031
32. 4,5.alpha.-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one Hydrochloride
33. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, Hydrochloride, (5.alpha.)-
| Molecular Weight | 337.8 g/mol |
|---|---|
| Molecular Formula | C17H20ClNO4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 337.1080858 g/mol |
| Monoisotopic Mass | 337.1080858 g/mol |
| Topological Polar Surface Area | 70 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 539 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Opana |
| PubMed Health | Oxymorphone (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg/ml; 5mg; 10mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 2 of 6 | |
|---|---|
| Drug Name | Opana er |
| PubMed Health | Oxymorphone (By mouth) |
| Drug Classes | Analgesic |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release is a semi-synthetic opioid analgesic supplied in 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, 30 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorpho... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Oxymorphone hydrochloride |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Avanthi; Teva; Mallinckrodt; Roxane; Actavis Elizabeth; Impax Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Opana |
| PubMed Health | Oxymorphone (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg/ml; 5mg; 10mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 5 of 6 | |
|---|---|
| Drug Name | Opana er |
| PubMed Health | Oxymorphone (By mouth) |
| Drug Classes | Analgesic |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release is a semi-synthetic opioid analgesic supplied in 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, 30 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorpho... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Oxymorphone hydrochloride |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 7.5mg; 30mg; 15mg; 5mg; 10mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Avanthi; Teva; Mallinckrodt; Roxane; Actavis Elizabeth; Impax Labs |
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21675
Submission : 2008-06-04
Status : Active
Type : II

NDC Package Code : 49812-0136
Start Marketing Date : 2012-06-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-02-15
Pay. Date : 2012-11-23
DMF Number : 22816
Submission : 2009-06-18
Status : Active
Type : II

NDC Package Code : 51634-0095
Start Marketing Date : 2009-06-17
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-02-12
Pay. Date : 2012-11-27
DMF Number : 14502
Submission : 1999-10-29
Status : Active
Type : II

NDC Package Code : 0406-0790
Start Marketing Date : 2010-01-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23403
Submission : 2009-12-22
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25628
Submission : 2011-12-22
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9109
Submission : 1991-05-07
Status : Inactive
Type : II

NDC Package Code : 0406-0790
Start Marketing Date : 2010-01-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7451
Submission : 1988-04-28
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23754
Submission : 2010-04-29
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
About the Company : Veranova is a global leader in the development and manufacturing of specialist and complex APIs for pharma and biotech customers. We have over 50 years of experience navigating the...
About the Company : Rusan Pharma is a fully integrated global pharmaceutical company specializing in the treatment of addiction & pain management. We manufacture & market a wide range of APIs & formul...
 Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
Noramco is a partner to the pharmaceutical industry for controlled substance development & manufacturing.
About the Company : Founded in 1979, Noramco specializes in the development and manufacturing of APIs for both opioid and non-opioid products. With expertise in controlled substance development and ma...
About the Company : Parand Daro Pharmaceutical Company is a knowledge-based company that commenced operations in 2015, focusing on the production of narcotic medicinal substances. Parand Daro holds th...
About the Company : Mallinckrodt Pharmaceuticals is a multibillion dollar specialty biopharmaceutical company focused on our mission: Managing Complexity. Improving Lives. We provide medicines to addr...

About the Company : OmnisMed Pharmaceuticals believes that good healthcare must be accessible to everyone; That is the core of our philosophy. We are experts in providing a wide range of high-quality ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Global Sales Information

Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 5MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 7.5MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 15MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 20MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 30MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 40MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET;ORAL
Dosage Strength : 5MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET;ORAL
Dosage Strength : 10MG
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : RX

Dosage Form : TABLET;ORAL
Dosage Strength : 5MG **Federal Register determination that ...
Price Per Pack :
Published in :
Country : USA
RX/OTC/DISCN : DISCN

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

Oxymorphone Hydrochloride +RC ARS
CAS Number :
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No : 4926

Noroxymorphone Hydrochloride CII (50 mg) (4,5...
CAS Number : 52446-24-9
Quantity Per Vial : 50
Sale Unit : mg
Price : $890.00
Details : Material Origin- Chemical Synthesis; USMCA- Y...
Monograph :
Storage :
Code/Batch No : Catalog #1473002 / R133E0

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
