
1. Numorphan
2. Opana
3. Oxymorphone Hcl
4. Oxymorphone Hydrochloride
1. Numorphan
2. 76-41-5
3. 14-hydroxydihydromorphinone
4. Oximorphonum
5. Dihydroxymorphinone
6. Dihydrohydroxymorphinone
7. Opana
8. 7,8-dihydro-14-hydroxymorphinone
9. Oxymorphine
10. (14s)-14-hydroxydihydromorphinone
11. Dihydro-14-hydroxymorphinone
12. Numorphone
13. Oxymorphone Cii
14. Morphinone, Dihydro-14-hydroxy-
15. Nsc-19045
16. Chembl963
17. 9vxa968e0c
18. Chebi:7865
19. Ids-no-003
20. Nih10323
21. Oxymorphone (inn)
22. Nih 10323
23. 3,14-dihydroxy-4,5-alpha-epoxy-17-methylmorphinan-6-one
24. Oxymorphone [inn]
25. Ossimorfone
26. Oximorfona
27. Oxymorphonum
28. Morphinan-6-one, 3,14-dihydroxy-4,5-alpha-epoxy-17-methyl-
29. Nsc 19045
30. (-)-oxymorphone
31. (4r,4as,7ar,12bs)-4a,9-dihydroxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one
32. (4r,4as,7ar,12bs)-4a,9-dihydroxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one
33. Ossimorfone [dcit]
34. Oxymorphone [inn:ban]
35. Oximorfona [inn-spanish]
36. Oxymorphonum [inn-latin]
37. Unii-9vxa968e0c
38. Hsdb 8060
39. Einecs 200-959-7
40. En3202
41. Brn 0041588
42. Oxymorphone [mi]
43. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, (5alpha)-
44. Oxymorphone [vandf]
45. Schembl3571
46. 4,5.alpha.-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one
47. Oxymorphone [who-dd]
48. Gtpl7094
49. Dea No. 9652
50. Dtxsid5023409
51. Oxymorphone Cii [usp-rs]
52. (5alpha)-3,14-dihydroxy-17-methyl-4,5-epoxymorphinan-6-one
53. Hy-b0618
54. Nsc19045
55. Oxymorphone 0.1 Mg/ml In Methanol
56. Oxymorphone 1.0 Mg/ml In Methanol
57. Zinc3875483
58. Bdbm50001707
59. Akos015962232
60. Db01192
61. Oxycodone Hydrochloride Impurity, Oxymorphone-
62. C08019
63. D08323
64. Q423380
65. 4,5-epoxy-3,14-dihydroxy-n-methyl-6-oxomorphinan
66. Morphinone, 7,8-dihydro-14-hydroxy- (6ci,7ci)
67. Oxycodone Hydrochloride Impurity A [ep Impurity]
68. 4,5alpha-epoxy-3,14-dihydroxy-17-methyl Morphinan-6-one
69. Morphinan-6-one,5.alpha.-epoxy-3,14-dihydroxy-17-methyl-
70. (5alpha)-4,5-epoxy-3,14-dihydroxyl-17-methylmorphinan-6-one
71. Morphinan-6-one, 4,5alpha-epoxy-3,14-dihydroxy-17-methyl-
72. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-
73. Morphinan-6-one, 4,5alpha-epoxy-3,14-dihydroxy-17-methyl- (8ci)
74. Morphinan-6-one,5-epoxy-3,14-dihydroxy-17-methyl-, (5.alpha.)-
75. Oxycodone Hydrochloride Impurity, Oxymorphone- [usp Impurity]
76. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, (5-alpha)-
77. Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-methyl-, (5alpha)- (9ci)
78. Oxymorphone Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
79. (1s,5r,13r,17s)-10,17-dihydroxy-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7(18),8,10-trien-14-one
80. 10,17-dihydroxy-4-methyl-(13r,17s)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10-trien-14-one
81. 10,17-dihydroxy-4-methyl-(13r,17s)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10-trien-14-one(oxymorphone)
82. 10,17-dihydroxy-4-methyl-(13r,17s)-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10-trien-14-one(xymorphone)
83. 10,17-dihydroxy-4-methyl-12-oxa-4-azapentacyclo[9.6.1.01,13.05,17.07,18]octadeca-7,9,11(18)-trien-14-one
| Molecular Weight | 301.34 g/mol |
|---|---|
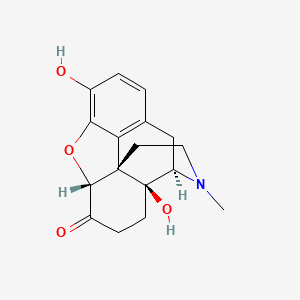
| Molecular Formula | C17H19NO4 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 301.13140809 g/mol |
| Monoisotopic Mass | 301.13140809 g/mol |
| Topological Polar Surface Area | 70 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 539 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Opana |
| PubMed Health | Oxymorphone (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg/ml; 5mg; 10mg |
| Market Status | Prescription |
| Company | Endo Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Opana |
| PubMed Health | Oxymorphone (Injection) |
| Drug Classes | Analgesic, Anesthetic Adjunct |
| Drug Label | OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per... |
| Active Ingredient | Oxymorphone hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg/ml; 5mg; 10mg |
| Market Status | Prescription |
| Company | Endo Pharms |
Adjuvants, Anesthesia; Analgesics, Opioid; Narcotics
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Oxymorphone injection is indicated for the relief of moderate to severe pain. It is also indicated for preoperative medication, for support of anesthesia, for obstetrical analgesia, and for relief of anxiety in patients with dyspnea associated with pulmonary edema secondary to acute left ventricular dysfunction. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
Oxymorphone injection is not approved for use in detoxification or maintenance treatment of opioid addiction. However, the history of an addictive disorder does not necessarily preclude the use of this medication for the treatment of chronic pain. These patients will require intensive monitoring for signs of misuse, abuse, or addiction. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
Oxymorphone injection should not be administered to patients with a known hypersensitivity to oxymorphone hydrochloride or to any of the other ingredients in oxymorphone injection, or with known hypersensitivity to morphine analogs such as codeine.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
Oxymorphone injection is contraindicated in patients with respiratory depression except in monitored settings and in the presence of resuscitative equipment and in patients with acute or severe bronchial asthma, upper airway obstruction, or hypercarbia. Oxymorphone injection is contraindicated in any patient who has or is suspected of having paralytic ileus.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
Oxymorphone injection should not be used in the treatment of pulmonary edema secondary to a chemical respiratory irritant. Opioid analgesics cause pooling of blood in the extremities by decreasing peripheral vascular resistance. This effect results in decreases in venous return, cardiac work, and pulmonary venous pressure, and blood is shifted from the central to peripheral circulation which would not be beneficial in the treatment of pulmonary edema secondary to a chemical respiratory irritant.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
Oxymorphone injection is contraindicated in patients with moderate and severe hepatic impairment.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
For more Drug Warnings (Complete) data for Oxymorphone (29 total), please visit the HSDB record page.
For the treatment of moderate-to-severe pain.
FDA Label
Oxymorphone is a semi-synthetic opioid substitute for morphine. It is a potent analgesic. Opioid analgesics exert their principal pharmacologic effects on the CNS and the gastrointestinal tract. The principal actions of therapeutic value are analgesia and sedation. Opioids produce respiratory depression by direct action on brain stem respiratory centers. The mechanism of respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension and to electrical stimulation.
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
N - Nervous system
N02 - Analgesics
N02A - Opioids
N02AA - Natural opium alkaloids
N02AA11 - Oxymorphone
Route of Elimination
Oxymorphone is highly metabolized, principally in the liver, and undergoes reduction or conjugation with glucuronic acid to form both active and inactive products. Because oxymorphone is extensively metabolized, <1% of the administered dose is excreted unchanged in the urine.
Oxymorphone is well absorbed following subcutaneous, im, or iv administration. Onset of action usually occurs within 5-10 minutes after iv administration and 10-15 minutes after subcutaneous or im administration. Analgesia is maintained for 3-6 hours following parenteral administration.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2201
Following oral administration of oxymorphone hydrochloride, bioavailability is approximately 10%. Following oral administration of oxymorphone hydrochloride conventional tablets with a high-fat meal, peak plasma concentrations and area under the concentration-time curve (AUC) were increased 38%.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2201
Following oral administration of oxymorphone hydrochloride extended-release tablets with food, peak plasma concentrations were increased 50%; AUC reportedly was unchanged in one study and increased 18% in another study. Bioavailability of oxymorphone was increased in patients with renal impairment, those with hepatic impairment, and in geriatric individuals following administration as extended-release tablets.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 2201
After an iv dose, the steady state volume of distribution was 3.08 +/- 1.14 L/kg in healthy male and female subjects.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
For more Absorption, Distribution and Excretion (Complete) data for Oxymorphone (12 total), please visit the HSDB record page.
Oxymorphone undergoes extensive hepatic metabolism in humans. After a 10 mg oral dose, 49% was excreted over a five-day period in the urine. Of this, 82% was excreted in the first 24 hours after administration. The recovered drug-related products contained the oxymorphone (1.9%), the conjugate of oxymorphone (44.1%), the 6(beta)-carbinol produced by 6-keto reduction of oxymorphone (0.3%), and the conjugates of 6(beta)-carbinol (2.6%) and 6(alpha)-carbinol (0.1%).
Oxymorphone is highly metabolized, principally in the liver, and undergoes reduction or conjugation with glucuronic acid to form both active and inactive products. The two major metabolites of oxymorphone are oxymorphone-3-glucuronide and 6-OH-oxymorphone.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
1.3 (+/-0.7) hours
Oxymorphone interacts predominantly with the opioid mu-receptor. These mu-binding sites are discretely distributed in the human brain, with high densities in the posterior amygdala, hypothalamus, thalamus, nucleus caudatus, putamen, and certain cortical areas. They are also found on the terminal axons of primary afferents within laminae I and II (substantia gelatinosa) of the spinal cord and in the spinal nucleus of the trigeminal nerve. Also, it has been shown that oxymorphone binds to and inhibits GABA inhibitory interneurons via mu-receptors. These interneurons normally inhibit the descending pain inhibition pathway. So, without the inhibitory signals, pain modulation can proceed downstream.
Oxymorphone is an opioid agonist whose principal therapeutic action is analgesia. ... In addition to analgesia, other pharmacological effects of opioid agonists include anxiolysis, euphoria, feelings of relaxation, respiratory depression, constipation, miosis, and cough suppression. Like all pure opioid agonist analgesics, with increasing doses there is increasing analgesia, unlike with mixed agonist/antagonists or non-opioid analgesics, where there is a limit to the analgesic effect with increasing doses. With pure opioid agonist analgesics, there is no defined maximum dose; the ceiling to analgesic effectiveness is imposed only by side effects, the more serious of which may include somnolence and respiratory depression.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
The precise mechanism of the analgesic action is unknown. However, specific CNS (central nervous system) opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and play a role in the analgesic effects of this drug. In addition, opioid receptors have been identified within the PNS (peripheral nervous system). The role that these receptors play in these drugs' analgesic effects is unknown.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
Opioids produce respiratory depression, likely by a direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
Opioids depress the cough reflex by direct effect on the cough center in the medulla oblongata. Antitussive effects may occur with doses lower than those usually required for analgesia. Opioids cause miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations
US Natl Inst Health; DailyMed. Current Medication Information for OPANA (oxymorphone hydrochloride) injection (February 2010). Available from, as of June 5, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=55639e61-8757-11de-8a39-0800200c9a66
For more Mechanism of Action (Complete) data for Oxymorphone (9 total), please visit the HSDB record page.